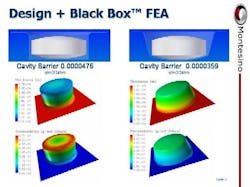
Blister packaging is a key component of pharmaceutical packaging globally as well as in the U.S. However, traditional or legacy pharmaceutical process control systems are not easily applied to blister packaging.At a time when the need for higher barrier packaging, and equipment speed and precision is growing exponentially, legacy tools are two-dimensional and of low resolution. Its a classic case of 20th century technology being used to address 21st century problems.Only by developing robust three-dimensional, high resolution processes and controls can blister packaging move to a more scientific basis and to a full and robust implementation of the principles of process analytical technology (PAT). Montesino aims to bring PAT to pharmaceutical packaging and has allied itself with several companies in both materials and equipment as it seeks to develop three-dimensional, high resolution services for pharmaceutical packaging. Prodieco Pharmaceutical Components (PPC) of Dublin, Ireland, a company whose focus has shifted from computer industry tooling to pharmaceutical tooling, introduced Montesino to 3D scanning, measuring and rendering and to a better understanding of the manufacture of high quality tooling for blister packaging. Amcor Flexibles licensed Montesino Black Box FEA simulation software for pharmaceutical blister packaging. A wide variety of materials and equipment companies including Honeywell are working with Montesino to increase the scientific base of blister packaging and move out of the dark ages into a three-dimensional, high-resolution universe.Legacy PackagingUntil very recently, blister packaging relied on legacy process controls best thought of as two-dimensional and of low-resolution. Material barrier data was primarily discussed in terms of flat or unformed sheet data, tooling and machine drawings were two-dimensional and of very low-resolution (typically, they did not even include tolerances or the other crucial specifications required in any engineering drawing). Process control tests were either compendial like USP 671 which requires time and often is quite "low resolution" in terms of what the data finally means or very limited like the methylene-blue vacuum leak testing for seal integrity. These tests offer neither the three-dimensional details nor the resolutions that would allow them to ensure package integrity. Two factors drove these conditions beyond their limits:
- A rise in the use of ICH accelerated stability testing conditions at 40°C, 65% relative humidity
- The development of new types of active pharmaceutical ingredients (APIs) and formulations that are highly sensitive to moisture and even to oxygen, UV, and other elements.
- Designing the blister pack correctly from the beginning, using robust specifications for minimum sealing areas and dimensions, cavity or pocket design, or types of materials to be used in the tooling, pharmaceutical packaging can reach a new level of precision and quality.
- Debugging that design using 3-D scanning tools, pharmaceutical packaging can use science to verify that the designed blister is actually the manufactured blister.
- And by using the rendering of that blister pack in an industry standard 3-D format, pharmaceutical packaging can deploy a design across multiple sites, multiple machines, even multiple geographical locations.
About the Author
Peter J. Schmitt
President
Sign up for our eNewsletters
Get the latest news and updates