Over the last several decades, there have been significant scientific discoveries in fields such as immunology, disease profiles (including various types of cancers), genetic sequencing, virology and more. The pharma industry has used these key scientific discoveries to develop novel therapeutics that target the specific nature of a disease to meet previously underserved or unserved patient populations. From mapping the first human genome in 2001 to delivering novel medical technologies based on gene modifications today, the industry has transformed at a pace never seen before in history.
Today’s new therapies are more sophisticated and more targeted in comparison to the blockbuster one-size-fits-all medications of the past. Whether a therapy is developed for a specific patient population, such as patients with a specific genetic marker, or a therapy is using cells captured from an individual patient (e.g., autologous cell therapy), smaller batch sizes are required to meet the scale of these targeted therapies.
Additionally, to circumvent the supply chain challenges felt worldwide starting in 2020, many governments and companies are adjusting their national policies and market strategies to ensure a secure supply by creating more regional or local manufacturing capabilities. This also means smaller batch sizes than the former blockbusters.
To adjust to these smaller scale needs, companies are building more multi-product manufacturing facilities. This includes both traditional biopharmaceutical companies, as well as contract development and manufacturing organizations (CDMOs). As part of the transformation driven by these product markets, the needs in the process development and manufacturing areas have also been intensifying and changing. These and other developments are calling for smaller batches, necessitating new solutions.
Planning a path forward
While modern trends drive the industry toward smaller batches, especially for new products, many of the traditional facility requirements to develop and manufacture these types of products remain. However, there are some noteworthy challenges in this market space. With many batches of smaller sizes and multiple products in a facility come business requirements for managing more recipes, records, changeovers, technology transfers, equipment, batches, materials, logs, data with context, and other factors.
Today’s most successful small batch manufacturers are categorizing these needs into three key strategic areas — capturing process knowledge, managing the transfer process and tracking the batches — while pairing the key needs in each of these areas with the associated enabling technologies to help them meet the increased demands of the small batch era.
Capturing process knowledge
Whether a facility is using experimental data from process development or data from a manufacturing process, making the contextualized data available at the right time for key decisions is critical. In the small batch world, the number of batches is significantly increased, which means that more data with context must be aggregated.
Capturing the knowledge about a product and defining the associated process is necessary for licensing. Yet, that same knowledge is also useful for developing new products with similar unit operations, or on the same platform, to share learnings from successes and failures, for transferring knowledge to support multiple manufacturing locations
And for managing the life cycle of the product (e.g., continued process verification, assessing deviations, evaluating potential changes, etc.). In the small batch world, these needs are intensified.
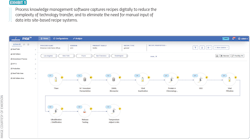
Several core technologies can be used to address these needs. Data collection, contextualization and aggregation tools are useful to make data available to scientists, operators, quality personnel and others in near-real time, with the ability to view data from experiments and batches across the globe. Process knowledge management solutions can be used for capturing and maintaining a master platform for storing manufacturing process flows, along with related data, such as critical process parameters (CPPs), critical quality attributes (CQAs) and other data sets (Exhibit 1).
Additionally, embedded in some of these solutions, or separately available, are quality risk management tools which are used to capture the relationships between CPPs and CQAs, along with the control strategies designated to manage potential risks.
Managing the transfer process
In cases where a process needs to be transferred, whether to the next stage in the life cycle, to/from a CDMO or to another internal facility, managing the manufacturing process, quality control process and associated parameter details (up to thousands of parameters for an individual product) is typically an extremely cumbersome manual process fraught with opportunities for errors.
Additionally, when transferring a new product to an existing facility, certain characteristics of the available equipment must be confirmed. Also, the ability to change out equipment and update systems easily — often referred to as flexible manufacturing — is important. Finally, when changing from one
product to another in manufacturing, structured processes and procedures need to be in place to ensure consistency and compliance.
Process knowledge management software provides these functions, along with the ability to export the pertinent manufacturing process and parameter details for use in the development of electronic and process automation recipes to enable faster technology transfer of the process and product. Additionally, this type of system can confirm that the equipment configured for a process meets the needs for a new product to be introduced in a facility, and it can highlight any areas where changes are needed.
Although some of the standards, such as NAMUR’s module type package, are yet to be finalized when it comes to flexibility or plug and play concepts, both suppliers (equipment and automation) and manufacturers have started to use the currently available technologies to make flexible manufacturing easier and faster. When it comes to ensuring that the process is properly connected and that the right equipment is in the right place for a specific product setup, electronic workflow tools can be used for confirmation.
Tracking the batches
In the smaller batch world, where many batches of different product types are produced in various manufacturing suites at the same time, it becomes even more critical to know details about the batches.
When an operator needs to perform an action on a batch, its location is important. Additionally, understanding the timing for performing a task and the status of the batch is key. Ensuring consistency in controlling the critical process parameters is also pertinent. The equipment status for all these smaller pieces of equipment located throughout the facility must be tracked, with material location and status relevant.
In the cases where a specific batch is designated for an individual, such as in CAR-T cell therapy, it is important to ensure that the operator is working on the right batch and documenting actions in the corresponding record. In this case, the chain of identity tracking throughout the entire manufacturing process must be maintained. Also, with many small batches come many batch records to be managed. Finally, many of these processes are paper- based, yet paper is inherently a particle shedder, complicating cleanroom operations.
Several technologies are being used to address the challenges that are common across the industry yet amplified in a facility producing many small batches. Real-time dynamic scheduling software is used to track the status of every batch and to help operators to know what to do when. These tools can also be used to evaluate the full extent or ripple effect of a delayed process step, or to determine the best time to perform maintenance on equipment. Additionally, the models from real-time dynamic scheduling software can be used to evaluate and eliminate bottlenecks.
And when used in conjunction with automation and electronic records, a scheduling system can automatically synchronize with the current state of those systems to minimize the number of manual scheduling-related tasks. Also, with process automation and electronic records solutions, the additional benefits of consistency in process controls, automated recording of key processing steps, reduced errors and elimination of paper in the cleanroom can be achieved.
With electronic records, technology solutions with varying degrees of complexity are available. If detailed tracking of materials, equipment and the process are necessary, the use of a comprehensive manufacturing execution system (MES) may be the best solution. However, for cases where recording of simple, straightforward tasks is required, less complex electronic workflow solutions can be used either instead of an MES, or in conjunction with an MES. Simpler solutions are often available in a software-as-a-service environment.
In the case of autologous cell therapy processes, there are several key features that should also be addressed in the integration of workflow, MES and scheduling solutions. At the manufacturing site, tracking the chain of identity for an individual patient’s batch includes receiving, storage, processing and shipping processes. During manufacturing, these solutions also include confirmation steps (e.g., sampling, testing, feeding, harvesting, storing, retrieving, preparing for shipping, etc.) that the batch under manipulation corresponds to the correct record with the right chain of identity reference, whether the recording process is paper batch records or electronic batch records or a combination of paper and electronic recording processes.
Solutions in action
Small batch facilities for process development, clinical manufacturing and commercial manufacturing are addressing many of these challenges with technology solutions. Clinical manufacturing facilities with a modular, agile design approach are using several of the aforementioned technologies — including process automation, MES and process knowledge management in conjunction with flexible recipe management — to enable on-demand delivery of treatments to patients in clinical trials. These systems can be easily reconfigured on demand and are future-proofed for new technology implementations.
As another example, a small batch equipment supplier for cell and gene therapy is using process automation solutions to orchestrate the overall process, and to collect data via this standard network, across several of their instruments.
Other examples include cell therapy manufacturers utilizing scheduling applications integrated with an MES to manage operations data for both electronic records and real-time shop floor activity-based scheduling; cell therapy manufacturing facilities utilizing an MES to manage equipment, materials, batch records and chain of identity; and gene therapy facilities utilizing equipment management and workflow capabilities to manage equipment log books and simple workflows associated with equipment activities.
Some of the benefits of applying these solutions include faster changeovers between products, rapid and more seamless transfer of products from one facility to another, reduced validation testing, review by exception, consistency in production operations, ease of use for operators across several unit operations, secure data storage and more.
With change comes innovation
In the small batch world, there are challenges with capturing process knowledge, managing technology transfer processes and tracking the many batches. But technologies exist to help manufacturers navigate these challenges. Although many of these challenges are also relevant for the larger batch world, most of them are intensified in the small batch market, and within that market there are some unique complexities.
As the industry continues to develop new therapies to meet the needs of patients, and as products along with their processes evolve, the associated challenges change. Yet technology solutions will continue to advance to meet these changing needs of the industry, ultimately continuing to support industry transformation.
About the Author
Michalle Adkins
Director, Life Sciences Strategy, Emerson
Michalle Adkins is director life sciences strategy and direction. She loves working in the life sciences world and has done so for over 30 years. She previously led the Emerson life sciences consulting team that used their varied experiences to work with several top pharmaceutical and biotech companies to provide consulting services for digital plant maturity assessments, future direction planning, solutions mapping, business justifications, and project definition. Prior to Emerson, she worked for Merck & Co., Inc. in various capacities including instrumentation, automation, and manufacturing, as well as vaccine scheduling and planning.
Ms. Adkins has a B.S. in Chemical Engineering and an M.E. in Industrial Engineering from The Pennsylvania State University as well as a Six Sigma Black Belt Master's Certificate from Villanova University.