How vulnerable is your pharma facility to natural disaster?
When an EF-3 tornado hit a Pfizer facility in North Carolina back in July, it caught the public’s attention when it was learned that the facility accounts for a quarter of Pfizer’s sterile injectables.
Recently, pharmaceutical supply chain disruption has been a major concern to the FDA and industry, amplified by the pandemic and rising geopolitical risk. Such black swan events — those so-called unknown unknowns lurking around the corner of every enterprise — are extremely difficult to assess until they occur.
Assessing the risk your facility has to natural disasters should be an essential part of your supply chain management process, and improved climate models and tools can help.
Natural hazard risk data
Extreme weather event risk is on the rise. Between 1980-2022, the National Atmospheric and Oceanic Administration (NOAA) determined that the U.S. averaged 8.1 $1 billion disasters per year — but that has risen to 18 events per year during the last five years.
Climate models and tools used to evaluate natural hazard risk have improved over the last decade, providing companies with the data and analysis to better understand their supply chain risk and resiliency to such events.
One useful source of data on natural hazard risk is provided by the Federal Emergency Management Agency (FEMA) National Risk Index (NRI). The data provides numerical assessments and risk ratings for 18 natural hazards in the U.S. taking into account expected annual losses from these events, community resiliency and social vulnerability. These risk ratings can be obtained at the census tract, county or state level.
To evaluate the natural hazard risk of U.S. pharma facilities, the FDA’s current drug registration site data were merged with the FEMA NRI data. The FDA site data used in the analysis includes information on the locations of U.S. facilities used to manufacture, prepare and process drugs.
A total of 6,047 such facilities were identified across the country. The NRI data provides five risk ratings, from ‘very high’ to ‘very low’ on the expected annual losses for each census tract associated with a hazard and each pharma facility address was mapped into a census tract. In the U.S., there are more than 84,000 census tracts.
Of the 18 hazards identified in the NRI data, the top risk affecting pharma facilities is from tornado damage where 37% of all facilities in the U.S. are in high-risk areas. Other high-risk hazards to pharma facilities include coastal and riverine flooding and wildfires.
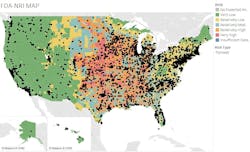
Figure 1 provides a visual representation of the distribution of U.S. pharma facilities by tornado risk. Each dot represents a facility with color-coding referencing the NRI ratings for tornadoes. The orange and red areas are concentrated in the center of the country where tornado activity is the greatest.
Table 1 provides more detail by state as to where pharma facilities are most exposed to tornadoes. Not surprisingly, Iowa (92%), Kansas (89%) and Illinois (86%) stand out as having the highest percentage of their facilities in relatively high or very high-risk areas.
Proactively addressing risks
This type of analysis can serve several purposes. First, it can be used as part of the process used to site a new facility. While the data presented here focuses on tornadoes, all hazards could be evaluated similarly and used to develop a composite hazard risk assessment.
Second, such analysis could be used as a starting point for a more precise risk assessment that can be obtained from catastrophe modeling of physical plant and hazards using detailed information on the size and features of the plant, geographical characteristics such as elevation and location in a flood plain, along with probability and severity of hazards affecting those facility sites. Such information could also be used to ‘harden’ facilities against natural disasters. A plant built a decade ago in a designated moderate flood risk zone using older flood maps might today be subject to greater risk of flooding as a result of updated flood models. Knowing that could help determine upgrades to critical infrastructure at the site that offer greater protection from damage.
Obtaining a baseline estimate of your facilities’ risk using the NRI data would help set the stage for a more in-depth assessment that can pay dividends over time in the form of lower insurance costs, out-of-pocket losses, downtime and other financial costs associated with a loss of use of the facility.
Extreme weather events appear to be rising in frequency and severity across the world. Pharma manufacturers are no less vulnerable to such events than any other company. Gaining insight into what hazards place pose the greatest risks to your facilities will place your company on a path to be better informed to proactively address these risks before they strike.