The Clock is Ticking: How to Speed up Efforts to Meet Traceability Deadlines
Starting November 2018, the U.S. Food and Drug Administration (FDA) will begin enforcing the requirement in the Drug Supply Chain Security Act (DSCSA) to include a product identifier on prescription drug packaging.2 The deadline for drugs coming from the EU is about two months later, on Feb. 9, 2019.
The regulation will help reduce drug counterfeiting, which is a significant problem in many parts of the world. In the U.S. and other developed countries, counterfeit drugs are only about 1 percent of the supply, but in developing countries they can be as much as 40 percent. Many of these counterfeits lack the therapeutic ingredients, have the right ingredient but at the wrong dose, or are tainted with toxic substances. This poses a significant health risk for patients, who have no way of telling the real from the fake.
For pharmaceutical manufacturers, counterfeit drugs pose a financial risk, as they eat away market share for the real medications. The new identifiers will help drug distributors distinguish between authentic drugs and fakes. While there is a definite benefit for both pharma and patients from using the identifiers, their use will introduce integration complexity and cost. Particularly for smaller companies, including generic drug manufacturers and contract manufacturing organizations, whose margins are extremely tight, traceability initiatives will be a challenge.
How ready are pharma companies in the U.S. and Europe to meet the deadlines?
Market research firm Penn Schoen Berland (PSB) recently surveyed 174 pharma companies, 155 contract manufacturing organizations, 50 wholesale distributors, 195 hospitals, 81 pharmacies and 11 third-party logistics providers to understand readiness of stakeholders to comply with the DSCSA and the Falsified Medicines Directive (FMD) in the U.S. and EU respectively.
Approximately 33 percent of the surveyed firms were confident of complying with basic requirements by the November deadline, such as shipment of serialized products, preparation of master data and resource allocation for driving the execution.1 But only 25 percent of the surveyed firms had carried out a serialization pilot, and only 11 percent were confident their CMOs would be ready to ship serialized products.
The deadline for FMD is two months after the U.S. deadline, and many European stakeholders are better equipped in terms of master data management readiness and projections of serialized units. Approximately 68 percent of pharma companies are somewhat ready, but only 15 percent of European companies have taken all measures to ensure serialization readiness. Also, the complexity of the EU market, which requires integration of pharmas, CMOs, wholesalers and retail pharmacies with the National Medication Verification System (NMVS) across 20 odd countries, is posing a major challenge for stakeholders with the approaching deadline.
If pharmas are to be ready by the deadlines, these organizations must accelerate their efforts now. Investment in hardware-agnostic serialization technologies, such as IoT, blockchain and machine learning capabilities, will be a key differentiator for pharmaceuticals companies. These solutions will not only help in meeting regulatory compliance but also help reduce production bottlenecks and optimize inventory utilization.
Beyond counterfeit protection, traceability will be key to success in precision medicine therapy
The availability of inexpensive genome sequencing has opened a window on the variation found within human diseases. Many conditions which were once identified as one disease are now recognized as different diseases with common symptoms but varying genetic causes. Instead of one blockbuster drug to treat all patients with a set of similar symptoms, pharmas are now developing a whole range of therapies targeted to each patient’s genetic makeup and physiology. These more precise treatments are targeted at much smaller populations, using patients’ own cells to create therapies with essentially a market of one.
With this new model of operations, pharmas will need to redesign manufacturing units to accommodate a quantum increase in the number of products produced and distributed. This approach will require more agile and responsive systems capable of making several products in a single day, while maintaining control and integrity of each product.
In this complex environment, digital track and trace technology will be critical to success. Without it, pharmas won’t be able to compete in the precision medicine market. But even in today’s market, track and trace technology can improve inventory management, allowing for more accurate product counts and real-time data on the location, storage conditions and expiration date of each unit.
Serialization will simplify global tracking
Anti-counterfeiting regulations apply only in a single country or group of countries. The FMD only governs drugs produced and distributed within the European Union and DSSCA is specific to the U.S., resulting in regional challenges in seamless tracking of counterfeit drugs across continents.
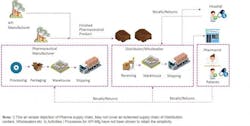
Serialization, which assigns a unique number to each saleable unit of a prescription product, links the product to information about its origin, batch number and expiration date. The units can then be tracked through its entire supply chain, including the origin of its active pharmaceutical ingredients (APIs), the specific batch in which it was created and the distributors who delivered it to the retail pharmacy for sale to the patient.
This ability to audit will separate the legitimate pharmas from counterfeiters and allow accurate tracking across all jurisdictions. It will protect pharmas and patients from counterfeits and, in the event of a quality problem, allow pharmas to recall only units that are affected.
Using blockchain and IoT technology to enable serialization
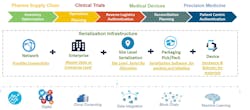
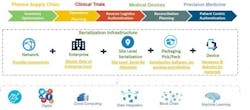
DSCSA calls for adoption of an interoperable tracking system by 2023. Two relatively new technologies will be key to achieving this tracking system: blockchain and the Internet of Things (IoT). Along with the infrastructure to support and connect all the components of the system, these two technologies will allow the high degree of visibility, traceability, reliability and interoperability that is needed. Figure 2 illustrates how these technologies can be integrated into life sciences operations.
IoT: Real-time visibility of location and conditions
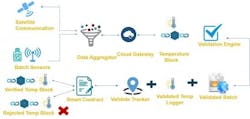
IoT technology enables instance-level visibility through a network of sensors to report on location and conditions. IoT can also enable real-time product authentication, as well as temperature monitoring and control during shipping.
The IoT solution is a closed loop system, comprising wireless or mobile connected sensors, a central database, dashboards, and business intelligence with a feedback loop. Data collected from IoT devices about a pharma product or medical device can also be fed into a business intelligence (BI) system or enterprise resource planning (ERP) system. These same devices can precisely pinpoint a product’s location within the supply chain to identify leaks and monitor chain-of-custody as the product moves through authorized channels and parties, which can ensure business processes are monitored and optimized. There are still some hurdles to cross to realize the complete “visibility” of supply chain through an IoT-enabled solution.4 Connectivity standards — particularly cross-border interoperability — are still not completely sorted out, and the cost and time of building the technology infrastructure can be high.
Blockchain is a public distributed-ledger system that tracks transactions, with each block of data added to an existing chain in a way that is permanent and unalterable.3 A blockchain-based system for monitoring could ensure a chain-of-custody log, tracking each step of the supply chain (along with additional data, such as the environmental conditions the product encounters) at the individual drug/product level, in a way that could not be corrupted.
Key advantages of adopting blockchain technology for a pharma supply chain traceability include:
- Data governance and records management. Accurate records are essential to verify conditions of contracts between wholesalers, distributors and pharma manufacturers are met.
- Counterfeit surveillance. With blockchain, every medicine can be tracked back to its origin and the information can be made available to all stakeholders through a secure transaction.
- Product quality assurance. With the chain of custody and environmental conditions verified, the pharma manufacturer, wholesalers, distributors and patients can be assured the product has not been rendered ineffective through improper handling.
- Regulatory compliance. Blockchain technology can provide accurate data for compliance with DSCA specific and FDA requirement.
The diagram above shows the standard roadmap for implementing a serialization and traceability solution using IoT, blockchain and cloud connectivity. The first step is to develop a sound business case that demonstrates the need for traceability of a specific product, showing the costs and potential savings from using the technology. Use cases will vary based on the specific needs of the organization, its financial situation, scale of implementation, regulatory challenges, etc.
Conclusion
Considering the numerous challenges in implementing serialization initiatives, a multi-pronged approach should be adopted by stakeholders such as pharma manufacturers, wholesalers, med device manufacturers and distributors in developing serialization and traceability solutions. Presently stakeholders will face data access and integration, storage and security issues along with regulatory restrictions imposed by the regional authorities. Using evolving technologies such as blockchain, IoT and cloud computing, stakeholders may need to create a framework for data interoperability and accessibility.
With the arrival of precision medicine, pharmas will need to document the chain of custody from a drug’s creation to the patient who uses it. Based on the proof of concept, a serialization architecture must be defined considering reusability and cost of implementation. The stakeholders will need to work closely with government and regulatory authorities to implement key technologies such as blockchain in serialization initiatives. Also, all solutions must be customized based on regional needs, capturing patient-centric data to drive end to end serialization, from the manufacturer to the patient.
[javascriptSnippet]
References:
1) Taylor, P. ( 2018, Jan 11) . Pharma 'may be overconfident' on serialization readiness. Retrieved from https://www.securingindustry.com
2) Drug Supply Chain Security Act (DSCSA)( Updated 2018, Feb 2). Retrieved from https://www.fda.gov
3) Iansiti, M. and Lakhani, K. ( Feb, 2018 issue). The truth about BlockChain. Retrieved from https://hbr.org/2017/01/the-truth-about-blockchain
4) Banafa, A.( 2017, March 21) 3 Major Challenges IoT is Facing. Retrieved from https://www.bbvaopenmind.com/en/3-major-challenges-facing-iot/