Empty chamber studies are an adaptation of loaded chamber temperature distribution evaluations described in FDA’s draft Large Volume Parenteral (LVP) CGMP regulation from 1976.i Constraining the temperature range across the loaded chamber has the goal of assuring sterilization is attained across the load, without over-processing of the filled containers. Section 212.244 of the draft regulation stated:
“The following factors shall be included in the design of sterilization processes: (a) Procedures required to establish uniform heat distribution in the sterilization medium of the vessel. Such procedures shall be capable of holding temperatures throughout the sterilizer to within ± 0.5°C or ± 1 °F from the time the product achieves process temperature until the heating portion of the cycle ceases.”
The intent of this expectation was to minimize the temperature variation across the sterilizing chamber during the sterilization dwell period so that the exposed product containers are neither under- nor over-processed. While intended for validation of LVP sterilization processes, expectations for this practice (and other LVP sterilization related concerns) were voluntarily accepted by the Small Volume Parenteral (SVP) industry in their haste to validate their own sterilization processes.
PDA’s Technical Monograph #1 published in 1978 drew upon the LVP regulation draft and described methods for the performance of Loaded Chamber-Heat Distribution studies.ii The initial validation protocols for steam sterilization included heat distribution studies with loads where thermocouples were positioned in the chamber external to the items rather than in the load itself. These were performed after empty chamber studies and prior to any microbiological challenge / heat penetration activities. These intermediate studies were assumed to be of importance because of their importance in terminal sterilization. The practices for loaded chamber temperature distribution studies mimicked the empty chamber effort and gave generally similar results. One of the original goals in this work was the identification of the ‘cold spot’, the location in the chamber where the temperature or F0 was the lowest and presumably presented potential difficulty in sterilizing the adjacent load items. Load items placed near this location were considered to be at greatest risk for lack of adequate sterilization. This initial belief included both terminal and parts sterilization.
Parts Sterilization
As steam sterilization validation became commonplace in SVP firms where terminal sterilization processes were minimally (if at all) performed, practices began to change. Differences in the individual load items in parts sterilization were revealed to play much a greater role in lethality than their physical location in the sterilizer. Since the goal was often the sterilization of many different items simultaneously, the focus on the ‘cold spot’ gradually shifted from the exterior of the load to locations within the individual load items that were most difficult to heat. That was the ‘cold spot’ of greatest concern rather than a chamber location. The configuration, size, orientation and wrapping of load items were increasingly understood to be of substantially greater impact on the sterilization process.
Given the similarity of results to that observed in empty chamber temperature distribution studies and lacking a formal requirement for their execution, many practitioners stopped performing the loaded chamber temperature distribution test and relied solely on the empty chamber results. This change in practice is increasingly prevalent in the SVP industry, as it was determined that the load items are more likely to affect the heat penetration and biological efficacy of the process than the external chamber conditions. Moving items within the chamber moved the ‘cold spot’ and it became evident that the load items had a substantially greater impact on the sterilization process than their position in the chamber. There were no meaningful ‘cold spots’ in the sterilization chamber, rather there were ‘slow to heat’ load items. Wherever those ‘difficult to heat’ items were placed in the load was the ‘cold spot’. This phenomenon was observed by numerous firms and elimination of loaded chamber heat distribution followed, but formal and documented evidence was not provided until Pavell and Hughes in 2010.
Absent a regulatory requirement for loaded chamber heat distribution in parts sterilization, and recognizing the difference between terminal sterilization (where all of the load items are identical) and parts sterilization (where load items vary in dimension, number, wrapping, etc.), loaded chamber temperature distribution should no longer be an element of parts sterilization validation. Table 1 outlines the potential results from a loaded chamber temperature distribution study for parts sterilization.
Study #
Physical Measurements from penetration locations
BI results in penetration
Physical Measurements from distribution locations
Acceptable Outcome?
1
>min F0
All killed
<±0.5°C
Yes
2
>min F0
Positivesa
<±0.5°C
No
3
All killed
No
4
>min F0
All killed
>±0.5°Cc
?
a – the use of overkill sterilization being commonplace, a surviving biological indicator would trigger an investigation
b - insufficient time at temperature or less than required F0 results would trigger an investigation
c - with satisfactory results from both physical and biological assessments of the process non-conformance of temperature distribution is non-critical and while reportable should not be considered reason for a deviation in the validation study.
The continued inclusion of the loaded chamber heat distribution test for parts sterilization during cycle development and validation is of limited value. The more difficult aspects of parts sterilization for porous loads relate to air and condensate removal and steam penetration. These are impacted to a far greater extent by the load items than by the conditions in the sterilizer chamber. The items’ mass (requiring more or less heat), orientation (for optimization of air / condensate removal), wrapping (assuring steam, air and condensate flow) and geometry (influencing the amount of internal air) have direct influence on the item’s sterilization. These are readily evaluated by the come-up or equilibration time. Variations in chamber conditions ordinarily do not alter the sterilization process and are best ignored.4Recognition that over-processing is also not a meaningful consideration for parts sterilization supports the elimination of an added, and largely superfluous, constraint on the temperature range with the load present. Empty chamber heat distribution studies serve as a convenient means for confirming initial sterilizer performance and as a rapid means for evaluating the effects of change on the system.
Terminal Sterilization
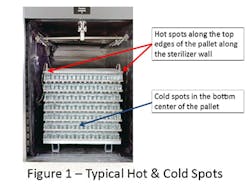
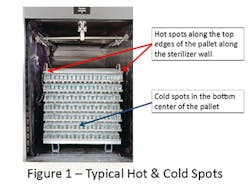
The sterilization problems that triggered the introduction of validation with the pharmaceutical industry occurred nearly 50 years ago.i,ii The important learning’s from those unfortunate events remain relevant today. When products are terminally sterilized in their final container, it is essential that the uniformity of the process across the sterilization process be achieved. The sterilized product containers must be both sterile and fully efficacious, neither under- nor over-processed, to assure patient safety. With the emerging interest in the use of lower temperature sterilizing conditions, maintaining a uniform process is increasingly important.iii Thus, while loaded chamber study requirements in parts sterilization have been gradually reduced, there has been no relaxation of the practice with terminal sterilization. It is important with terminal sterilization to begin with empty chamber studies and then to evaluate loaded chamber temperature distribution with loads. As terminal sterilization loads are comprised of a single container size filled with a constant volume of the same liquid, the individual containers respond to the external conditions in a consistent manner. The sameness of the load items means that chamber conditions play a major role in assuring uniformity of the delivered process. Thus, higher lethality is common in the upper part of the load, and the coldest areas of the load are typically found in the center bottom of the load (assuming a dense pack as shown in Figure 1).
The exact locations of the ‘hot’ and ‘cold’ spots in the chamber will vary with the sterilizer load and sterilizer design and should be determined during the cycle development phase of the validation effort. The ‘hot’ and ‘cold’ spots should be understood to be a zone rather than an individual container, with containers in that zone behaving similarly.
To assure uniformity of sterilization efficacy over time the chamber conditions must be maintained within tolerances established during the cycle development for loaded chamber temperature distribution. In well designed and constructed terminal sterilizers the variation across the chamber with the sterilizer could be as little as ±0.3 °C. It should be understood that the loaded chamber temperature distribution is not constant from beginning to end of the sterilization cycle dwell. It will be greatest at start of the dwell period, and gradually improve. The tightening of the temperature range is the result of the filled containers attaining the set-point temperature and stabilization of the chamber conditions. The loaded chamber temperature distribution practices must consider the duration of the data collection. The vagaries of container and load size suggest that data from the last few minutes of the dwell period is perhaps most indicative of sterilizer performance.
From a sterilization perspective, the range of temperatures during the process may be less critical than might be expected; after all it will certainly change during the process duration. Minimizing the lethality range delivered to the containers across the load is of far greater importance, and this is customarily assessed using F0 determinations derived from penetration temperatures. The lethality variation across the load should be minimized to balance the competing needs for sterilization (where high F0 is desirable) and stability (where low F0 is desired). How this balance is attained relies on more than just the heat distribution across the chamber. It must be understood that the lethality range is influenced as much by the load and container size as it is by the sterilizer process and design. Thus establishment of a singular expectation for the lethality range for all terminal sterilization applications is impractical. The goal should be to minimize the difference between the extremes to the extent possible.
To ensure stability of heat-labile products it is generally preferable to minimize the dwell time at elevated temperatures. One way to accomplish this is to shorten the overall cycle as much as possible while preserving sterilization effectiveness (i.e., uniform F0 across the load). Because lethality is accumulated during both the heating and cooling portions of the load these phases alone might deliver the required lethality, while minimizing the high-temperature dwell period duration. In such cycles, the steady-state conditions become less important to the sterilization process, but may still impact product stability. Consequently, empty chamber temperature distribution studies which avoid any load, container and process influence are perhaps more useful in confirming sterilizer performance.
Conclusion
The origins of validation in the United States can be traced back to the FDA’s proposed LVP regulation in 1976. Elements of that document are still evident in today’s validation practices. Experience has taught us that we must remain open to new understanding. The original intent of loaded chamber heat (temperature) distribution studies was in assuring patient safety for terminally sterilized products. The adoption of this practice for non-terminal moist heat sterilization was perhaps one of expedience, rather than considered science. As experience was gained, the necessity for this type of study with varied load items was questioned and gradually changed. The limited insight that the healthcare industry had in relation to parts sterilization has changed over time and practices have largely been revised to reflect newer understanding.
Expectations for loaded chamber heat distribution studies for terminal sterilization processes are likely to persist for years to come. Nevertheless, with the increasing sophistication of sterilizer designs and the generally superior performance now available, heat distribution tests are an artifact of the past rather than a truly useful contemporary tool. The variation of lethality between the hot and cold spots is a far more appropriate means for evaluating sterilizer performance.
[javascriptSnippet]