Differential Scanning Calorimetry Finds a Place in Manufacturing
The stability of a protein is one of the critical factors influencing its safety and efficacy as a potential biotherapeutic. Consequently, a variety of analytical techniques are deployed in determining and monitoring protein stability throughout biopharma development and production processes. Of these, differential scanning calorimetry (DSC) has long played a role in preformulation development. Now, DSC is expanding into the manufacturing environment, offering significant gains in productivity. This article looks at the technique’s contribution to biopharma development and explores the advances in technology and regulatory compliance that are facilitating its migration into the manufacturing space.
Differential scanning calorimetry (DSC) is regarded as the gold standard technique for characterizing the thermal stability of a protein, a key attribute that determines the molecule’s inherent stability. Traditionally, DSC has been used predominantly at the preformulation stage, where its value as a highly discriminatory and representative method for assessing stability is well-established. DSC data routinely informs decisions from candidate selection through to critical aspects of liquid formulation, such as excipient and preservative selection, ideal buffer characteristics and pH.
An area of emerging DSC application is in manufacturing support. As a sensitive detector of even very small differences in higher order structure (HOS), DSC is becoming increasingly recognized as a powerful technique for monitoring changes or deterioration in the structure of a protein. This makes it valuable for checking batch-to-batch comparability and in quality control.
A logical next step is to transfer DSC directly into the manufacturing space, to verify that HOS is maintained through to the point of product release. Advances in analytical capabilities and in the regulatory compliance “readiness” of DSC systems are making this an achievable goal, with robust method development and method transfer capabilities further supporting reliable data comparisons, both within and between manufacturing sites.
Focus on Protein Stability
To better understand how DSC supports biopharma development workflows, both conventionally in preformulation and now in manufacturing, it’s useful to consider the nature and importance of protein stability, and to look at DSC as a technique.
Inherently stable proteins are widely desirable as developable drug candidates, whether that stability occurs naturally or through engineering. Compared with less stable alternatives, such proteins are more likely to retain functionality during formulation and storage, to exhibit fewer tendencies to aggregate, and to present fewer problems during manufacturing.
The stability of a protein is directly impacted by its structure. A protein’s primary structure is the linear sequence of amino acids that form the polypeptide chain; beyond this, a protein has a three-dimensional form or HOS which encompasses secondary, tertiary and quaternary structures. These directly influence protein functionality and are critical to the quality, safety and efficacy of biopharma products. In Quality by Design terms, the stability of a candidate molecule is studied as part of HOS analysis to understand and control the molecule’s characteristics. This creates an associated need for analytical techniques that can characterize HOS throughout development and into manufacture.
Understanding DSC
DSC measures the heat absorbed as a protein denatures under the influence of increasing temperature, detecting the heat changes associated with hydrogen bond breakage as the structure unfolds. DSC is a universal technique that can examine the unfolding of all proteins (and other macromolecules) and all domains, and it requires no labels nor probes of any kind, allowing the examination of samples in their native state.
In practice, DSC involves measurement of the excess heat capacity (Cp) of a sample as a function of temperature. The thermal core of the calorimeter consists of a sample cell which holds the protein in solution, and a reference cell containing a matched buffer, both cells being housed within an insulating jacket. The two cells are maintained at the same temperature and, during a measurement, are heated at a constant scan rate. As the sample molecule unfolds, absorbing heat, a temperature difference (ΔT) is created between the cells, inducing a thermal gradient. The instrument automatically returns ΔT to 0°C by increasing power input to the sample cell, with power input subsequently converted into Cp values.
DSC in the biopharma development pipeline
In preformulation, and sometimes even earlier, DSC is used primarily as a simple screen for thermal stability (Tm). Tmvalues have been shown to correlate well with the longevity and shelf-life of a protein and, while their measurement does not remove the need for long-term stability studies, results support initial decision-making around candidate selection and formulation optimization. At this stage, DSC is one of the stability testing techniques used to test drug candidates under differing conditions of buffers, excipients and pH, to determine the most effective solutions for further development.
As a candidate molecule progresses through development, more detailed characterization becomes crucial and it’s the sensitivity of DSC to changes in tertiary and quaternary structure that arguably becomes most informative. During biotherapeutic development and manufacture, a protein may be stressed and liable to denature at multiple points, including during purification, when it is removed from conditions under which it is stable and active, and in production and formulation, where exposure to heat, chemicals, pH changes, pressure, mixing, and different material surfaces may result in denaturation. Furthermore, denaturation or modification may lead to aggregate formation, potentially reducing therapeutic efficacy and risking an immune response in the patient.
Throughout production, great importance is therefore placed on demonstrating that a protein drug is comparable in attributes such as structure, stability and size distribution to: previous lots of the same protein, and also the reference protein lot; the same protein manufactured at different sites; the same protein manufactured using modified processes or after process scale-up; or possibly the same protein in a different formulation. Changes to the manufacturing process may take place in process development or after drug approval, and for a variety of reasons. These may include improvements to manufacturing processes; increased scale; site change; compliance and regulatory change; or product stability improvements. In every case, it’s essential to verify that such changes do not impact the stability and HOS structure of the protein.
DSC is complementary to a range of other techniques that are applied in HOS fingerprinting/comparability studies, to confirm the structural consistency of the protein. These techniques include, but are not limited to: dynamic light scattering; fluorescence; circular dichroism; size exclusion chromatography; Raman spectroscopy; nanoparticle tracking analysis; resonant mass measurement; and microscopy. In combination, they support the optimization of process conditions towards the goal of maximizing yield by the most cost-effective method, and enable robust batch-to-batch comparability testing both pre- and post-drug product approval.
It’s in this latter manufacturing support role that DSC is emerging as particularly valuable in delivering reproducible, non-subjective stability comparability data, whether for quality control or for establishing biosimilarity. Until recently, though, the practicalities of DSC systems have meant that this valuable data generation could not be taken to the next step and applied directly in the GMP manufacturing environment. The advent of fully automated, 21 CFR Part 11 compliance-ready DSC systems able to characterize samples at therapeutic concentrations directly addresses this limitation.
Moving DSC Into Mfging
Establishing DSC in manufacturing support, and introducing it as a capable analytical tool for the GMP environment, has required the further evolution of DSC technology from its secure platform as an established lab-based technique. Challenges are focused around the requirements for easy-to-use, cost-effective and automation-ready instrumentation and, critically, the need for regulatory compliance. Advances in these areas deliver benefits at every stage of biopharma development and now make at-line measurement in manufacturing a viable option.
The latest DSC systems (exemplified by MicroCal PEAQ-DSC, Malvern Panalytical) are designed specifically for use in regulated environments, from GxP laboratories to GMP manufacturing. Controlled access to specific features, electronic signatures, auditable reports, and the facility to centralize data, provide the building blocks for compliance with 21 CFR Part 11. Increased automation, intelligent non-subjective analysis, robust method development and method transfer all contribute to greater productivity, consistency and applicability in manufacturing.
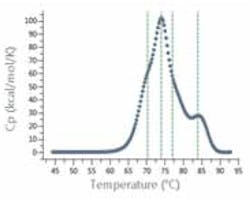
Figure 1: Analysis software automatically detects shoulders for analysis of multi-domain proteins, such as antibodies.
Such systems significantly boost the precision and value of DSC comparability assays, which are designed to demonstrate that the protein is “highly similar” to a reference molecule in terms of designated CQAs. Advanced DSC systems include software that eases SOP-driven method development and the subsequent transfer of knowledge, methods and data between individuals, departments and sites. This supports users in ensuring that all experiments are performed in exactly the same way (and that analysis methods are the same), when comparing data. The greater consistency and integrity of the data generated in such an auditable manner adds strength to comparability studies and confidence for submissions to regulatory bodies.Adding further weight is fully automated data analysis and algorithms that allow highly discriminating comparison of thermograms, identifying even subtle shoulders in peaks, and allowing, for example, non-subjective identification of the stability of a sub-domain (Figure 1).
Software advances also enable the simultaneous analysis of multiple data sets, so that analysis is faster and variability arising from user subjectivity is eliminated. This increases the robustness of comparability studies, whether batch-to-batch or when developing biosimilars. Quantitative comparison tools can now compare Tm, enthalpy and shape, where shape comparison looks at subtle differences in relative peak height and shape, comparing batches of a multi-domain protein. This quantitative comparability makes DSC more sensitive to changes in higher order structure by accessing more of the data generated. It also makes it possible to set pass/fail criteria (Figure 2).
Figure 2: Quantitative comparison tools compare Tm, enthalpy and shape, and allow setting of pass/fail criteria.
What’s equally important, from the perspective of manufacturing support, is that the new availability of regulatory compliance tools in DSC systems means that those methodologies that have proven so powerful for HOS analysis in quality control can now be transferred into the manufacturing space. Without those tools, alternative analyses and methodologies have to be developed. The transfer of protocols and data analysis can now take place throughout the biopharmaceutical development process and into commercial production. This reduces the time and costs associated with developing new analytical methods and, significantly, it means there are now DSC systems that can be used at-line for protein stability characterization.
Powerful Technique
Prized in preformulation for its ability to generate data that correlates well with long-term stability, DSC is also a powerful technique for manufacturing. Highly sensitive to changes in HOS, especially with tertiary and quaternary structure, it can be used alongside other techniques to build a robust and valuable HOS fingerprint for comparability studies. Advances in DSC make analysis faster and easier, while eliminating the subjectivity associated with comparative studies.
Such advances make it easier to determine whether or not a protein is sufficiently similar to a reference to meet quality control or biosimilarity targets, and are accompanied by features that facilitate the use of DSC within the manufacturing environment. In this way, modern DSC systems expose the full capabilities of the technique as a stability assessment tool, from screening and preformulation, through to at-line measurement for direct manufacturing support.
[javascriptSnippet]