Once a drug candidate has been selected, the ability to rapidly scale-up production is critical in the business and clinical timeline. Providing materials for late-phase clinical trials and product launch directly affects time to market. New platforms and research have drastically improved this transition, however, the period between the end of Phase II studies and the scale-up for Phase III and product launch creates a new biotech valley: the “Valley of Financial Pain.” Time to market may be the difference in success or failure of a product.
The iBio facility is designed to manufacture up to 300 kg of pure monoclonal antibodies per year using hydroponic, vertical farming.
The manufacturing of CHO (Chinese hamster ovary)-made pharmaceuticals can be expensive, time consuming and laborious to scale-up from benchtop to manufacturing scale bioreactors. Cell lines have a relatively long development period and are often at risk of contamination by adventitious agents. The scale-up of stirred-tank bioreactor production requires additional engineering and cell growth optimization work at each step. CHO-made products produce recombinant proteins with heterogeneous glycosylation profiles. In most cases, stabilization of these glycoform profiles needs to be optimized by extensive, time-consuming cell growth studies.THE BIOREACTOR IS A SINGLE PLANT
Plant-based transient expression systems allow a direct scale-up from bench to manufacturing without extensive comparability studies since the bioreactor unit is a single plant and scale-up is assured by growing more plants instead of building larger bioreactors. They offer an alternative to traditional mammalian cell culture or plant transgenic technologies.
The development of transgenic plants is a time consuming process that can take years. Historically, yields in transgenic plants have been lower than new transient systems. There are legal, political and social concerns surrounding the containment of transgenes being released into the environment. While there are regulations surrounding these issues, the use of transgenic plants to make biopharmaceuticals in “field-grown” conditions is not prevalent.
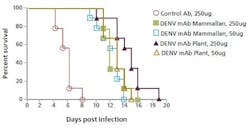
The plant-made anti-dengue virus antibody significantly increased the survival time of infected mice compared to those treated with the CHO-based product.
Plant transient expression classically expresses multiple proteins at the same time, through Agrobacterium sp. based vectors into the plant cell by vacuum infiltration. The plant vacuum infiltration transfection procedure is a scalable process that eliminates the need for the generation of a stable mammalian or plant cell lines to produce therapeutics at commercial scale. Transient expression systems offer the advantage of expressing and recovering protein within a few days after transfection.This system allows a direct scale-up from bench to manufacturing without extensive comparability studies. The bioreactor unit is a single plant and scale-up is assured by growing more plants instead of building larger bioreactors. Over the years, the founders of iBio CMO LLC have developed and implemented the agroinfiltration technology to manufacturing scale, providing a flexible and more affordable alternative to current manufacturing practices.THE CHALLENGE
United Therapeutics approached iBio CMO with a challenging project — to manufacture pre-clinical material of three anti-viral antibodies in less than two months — from gene to released product, and compare them to a CHO-made counterpart, in a mouse survival study.
United Therapeutics understood that if the team could successfully complete the plant expression manufacturing process on a small scale, then it would be easily transferable to the large-scale manufacturing facility where over 2.2 million plants can be grown to produce 10-20 kg of purified plant-made therapeutics or vaccines per month for clinical studies.
The iBio CMO facility showcases some remarkable features. The 139,000-square-foot manufacturing building is located in Bryan-College Station, Texas, home of Texas A&M University. The facility is designed to manufacture up to 300 kg of pure monoclonal antibodies per year using hydroponic, vertical farming to reduce foot print and increase quality control over plant growth.
A plant-based expression platform offers substantial technical advantages over traditional expression systems such as: flexibility (in scale and production), rapid manufacturing, sustainability, simple upstream process, comparatively low cost of goods and rapid time to market producing both biotherapeutics and vaccines.
The pilot scale facility and manufacturing facility are all under the same roof to facilitate technical transfer protocols.
EXECUTION
There were three specific goals:
1) To produce pre-clinical material of three anti-viral antibodies within a two-month deadline
2) To release pre-clinical material for animal studies
3) To compare plant-made and CHO-made pharmaceuticals production time frame and efficacy
The iBio CMO facility received the protein sequences from United Therapeutics and in three weeks produced a codon optimized vector to transfect plants. The vector was amplified in two days and plants were transfected by vacuum infiltration. The upstream steps in plant-expression do not require sterile conditions.
Production of the target protein takes between five and seven days post-infiltration. There is no permanent genetic modification of the plant in this transient system. The resulting tissue is homogenized and the proteins are extracted. Procedures are in place for both hydrophilic and hydrophobic proteins. The extract then moves to downstream purification by filtration and chromatography systems typical for protein biotherapeutics.
The pilot scale facility and manufacturing facility are all under the same roof to facilitate technical transfer protocols.
At pilot scale it is typical to produce gram quantities in weeks. The process development effort is always centered around one plant species. Multiple mammalian cell lines are often used for discovery and then processes are transferred to production cell lines. This is not necessary with plant production; the bioreactor is always the same and the transfection system is always the same. This provides great confidence that scale-up from pilot to manufacturing scale will be seamless. One never changes the scale of the bioreactor; it always remains a single plant.RESULTS
iBio CMO produced three anti-viral antibodies between 90 percent and 95 percent purity within two months. All antibodies were glycosylated, and revealed a consistent glycoform pattern: one major glycoform and two to three minor glycoforms, which is important. A stable glycosylation pattern means a straightforward scale-up to commercial production.
In the literature 1,3-fucosyl and b1,2-xylosyl knockout plants have resulted in high glycosylation homogeneity and exhibited 20- to 35-fold increase in biological activity compared to its CHO-produced counterpart (Cox et al., 2006).
In this case, plant-made products are tested against a CHO-made counterpart in a controlled mice survival study. A comparison was made for the antibodies’ efficiency at protecting the mice infected with the dengue virus in a side-by-side trial. The plant-made anti-dengue virus antibody significantly increased the survival time of infected mice compared to those treated with the CHO-based product (see chart). Research continues to determine the mechanism of this improvement.
Defined goals were achieved by demonstrating the production of monoclonal antibodies from gene to protein in three weeks, and about 1 gram of pre-clinical material was purified and released in five to six weeks. The transient expression of a selected anti-viral antibody can be further manufactured to kg quantities within two to three months, if required.
FINDING THE RIGHT SOLUTION
iBio CMO’s platform provides solutions for scientists and pharmaceutical manufacturers experiencing problems in producing biotherapeutics or vaccines.
Using the transient plant expression production platform, biotherapeutics or vaccines for pre-clinical or early clinical stages can quickly be generated from gene to protein within a few weeks and can be scaled-up for large-scale manufacturing within a short period without affecting the quality of the product. This system also has tremendous capability to provide rapid countermeasures to outbreaks and national biological threats by ramping up large-scale production of a vaccine or other biothereapeutics in a short time period.
Scientists can quickly and economically test the transient plant-based expression as an alternative expression system in parallel to mammalian, insect and bacterial cell expression systems for customized biomolecules rapid production. Plant transient technology can also be utilized to produce proteins that are difficult to produce. For manufacturers, it is a cost effective method for screening drug candidates and getting answers quickly with the knowledge that large-scale facilities are already built and functional for scale-up.