Vials, ampules, syringes and rubber stoppers are just a few examples of primary packaging components that pharmaceutical drug manufacturers utilize to package their end products. When these components are utilized for injectable drug products, also known as parenteral drug products, they must undergo several discrete processes allowing them to meet or exceed standards developed by the United States Pharmacopoeia (USP) and enforced by the Food and Drug Administration (FDA). The two critical processes are particulate cleaning and depyrogenation.
Particulate cleaning is a pre-sterilization process that reduces the amount of sub-visible particles that can be found in packaging components. The test methods and limits for sub-visible particles are outlined in USP chapter <788> titled “Particulate Matter in Injections.” Depyrogenation is a dry heat process that reduces the amount of bacterial endotoxins in packaging components. The test methods and limits for bacterial endotoxins are outlined in USP chapter <85> titled the “Bacterial Endotoxin Test.”
Particulate Cleaning
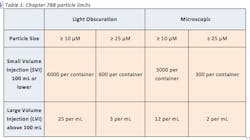
Particulate matter in injections can be defined as extraneous, mobile, undissolved particles, unintentionally present in the end product (3). These contaminants can come from several sources such as the environment, packaging materials and formulation ingredients (5). Particulate matter can be extremely harmful when introduced into the bloodstream and can cause several adverse reactions in the patient such as vein irritation, local tissue infarction, anaphylactic shock and even death. Therefore, the USP places limits on the amount of sub-visible particles that are allowed in injections. The USP’s limits on particulate matter, harmonized with the European Pharmacopeia and Japanese Pharmacopeia, are outlined in USP Chapter 788 “Particulate Matter in Injections.” The chapter contains two test methods for particulate matter which are the Light Obscuration Particle Count Test and the Microscopic Particle Count Test. These two methods are utilized to determine the amount of sub-visible particles in the final product of small-volume injections (SVI) and large-volume injections (LVI). The details of the limits outlined in 788 are found in Table 1: Current 788 Particle Limits.
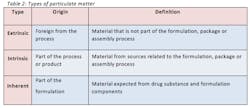
In general, any semi-solid to solid material may be counted as a particle and may be considered hazardous upon identification (5). Examples of such particles are air, liquid, gel, singular solid, aggregate, agglomerate, drug solid, salt, polymorph, lubricant and plasticizer. Three common types of particulate matter are extrinsic, intrinsic and inherent, described in table 2. It is critical to fully understand the identification, origin and characterization of particulate matter so the final product meets or exceeds the allowable particle count test for USP 788.
When utilizing packaging components for parenteral drug products, it is critical to reduce particulate matter to acceptable levels through USP purified water and Water for Injections (WFI) rinses. A typical procedure is outlined below.
- Sterile air blowing
- Three US Purified water rinses
- One final rinse with Water for Injection (WFI)
- Final air blowing
It is important to note that the cleaning must be done in a certified class 100/10 clean room with high-efficiency particulate HEPA filtering. HEPA are air filters that must satisfy specific standards of efficiency and remove 99.97% of particles that have a size larger than 0.3 μm from the air that passes through.
Depyrogenation
Depyrogenation can be defined as the reduction of pyrogenic substances, including bacterial endotoxin, and is generally achieved by removal or inactivation (5). Specifically, bacterial endotoxins are lipopolysaccharides which are molecules found on the cell wall of gram-negative bacteria. Lipopolysaccharides are relatively large molecules that are thermally stable and insensitive to pH changes (7). Therefore, destroying these molecules tend to be quite difficult. However, it is critical to reduce these to acceptable amounts when packaging parenteral drug products due to their harsh effects when injected into the body.
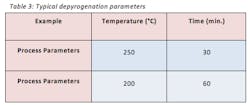
There are several techniques in which depyrogenation can be achieved. The two most common depyrogenation techniques are dry heat via a depyrogenation oven that reaches temperatures as high as 250°C or through a series of rinse cycles that consists of washing and rinsing with USP purified water and water for injection (WFI). The selection of technique is dependent on the chemical characteristics that make up the packaging component. For example, most glass vials are made from borosilicate glass which has a softening point of roughly 820°C; therefore, introducing a packaging component made up of borosilicate glass to a depyrogenation oven at a temperature of 250°C would not cause any issues. On the other hand, a rubber stopper can degrade at temperatures much less than 250°C; therefore, a rubber stopper would be depyrogenated via a series of cycles of washing and rinsing with USP purified water and WFI. It is important to note that both techniques require a validated three-log reduction in endotoxins starting from a concentration of at least 1000 EU.
A depyrogenation tunnels usually consist of three chambers. The first chamber is the “inlet chamber” that emits HEPA filtered air. This laminar flow keeps the transfer of glassware clean and protects the product from the potential “hot air back flow” coming from the hot chamber. The second chamber is the “hot chamber” where the glassware goes through its depyrogenation cycle. The third chamber is the “cooling chamber” which cools the glassware to room temperature so it can be sent to a sterile environment with Class 100/Grade A/ISO 5 conditions. Table 3 displays common depyrogenation parameters in a batch oven or continuous tunnel.
Rubber stoppers are also a potential source of bacterial endotoxins. Due to their chemical characteristics, they are usually depyrogenated through a series of washing and rinsing cycles prior to the final steam sterilization procedure. It is important to note that the final rinse needs to be with WFI, and it is important to minimize the lapsed time between washing and sterilizing as moisture on the rubber stoppers can support microbiological growth.
Bacterial endotoxins are strongly regulated and strictly tested for in regards of parenteral drug products. USP chapter <85> titled “The Bacterial Endotoxin Test” outlines three test methods for bacterial endotoxins. The three test methods are the gel-clot method, the turbidimetric method and the chromogenic method. Although these three methods are different, they are based on the same principle which is the fact that bacterial endotoxins react with Limulus Amebocyte Lysate (LAL).
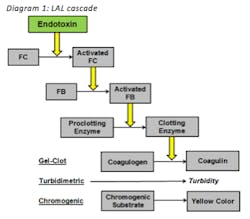
LAL is an aqueous extract of blood cells from horseshoe crab. Diagram 1 displays the overall cascade. When bacterial endotoxin reacts with Factor C it becomes activated which activates sequential enzymes within the cascade. Ultimately the proclotting enzyme turns into the clotting enzyme which causes the coagulen to turn into coagulin. Coagulin is the protein from horseshow crab blood that forms a gel-clot when reacted with bacterial endotoxin. This is the basis behind the gel-clot method. For the turbidimetric method, a turbidimetric assay is introduced within the reaction which causes turbidity if bacterial endotoxins are present. This is the basis behind the turbidimetirc method. For the chromogenic method, a chromogenic substrate is introduced to the reaction. If bacterial endotoxins are present the substrate is cleaved and a yellow color is produced. This is the basis behind the chromogenic method.
As you can see all three methods are based on the same principle which is the fact that bacterial endotoxins react with LAL; however, they differ in the last couple of steps.
Conclusion
Particulate cleaning and depyrogenation are critical processes that when validated reduces sub-visible particles and bacterial endotoxins to acceptable levels. There are many factors to consider when performing these procedures such as the chemical characteristic of the packaging component, cycle time of the specific process and intended use of the packaging component. These are just a few of the many considerations to take into account when packaging parenteral drug products. Yet, with a complete understanding of processes and test methods, the final drug product will not contain any contaminants that could harm the end-user.
References
- Gecsey, J., & Harrison, T. (2005). Sampling and preparation techniques key to success in meeting new requirements for particulate analysis in SVPs. European Journal of Parenteral & Pharmaceutical Science, 10(3), 79 82.
- General Chapter <1> Injections. USP 36/NF 31: United States Pharmacopeia. www.usp.org
- General Chapter <788> Particulate Matter in Injections. USP 36/NF 31: United States Pharmacopeia. www.usp.org
- Langille, S. E. (2013). Particulate Matter in Injectable Drug Products. PDA Journal of Pharmaceutical Science and Technology, 67(3), 186-200.
- Singh, S. K. (2013). Particulate Matter in Sterile Parenteral Products. In P. Kolhe, M. Shah, & N. Rathore (Eds.), Sterile Product Development: Formulation, Process, Quality and Regulatory Considerations (Vol. 6, Advances in the Pharmaceutical Sciences, pp. 359-409). AAPS.
- Williams, Kevin L. Endotoxins: Pyrogens, LAL Testing and Depyrogenation. 3rd ed. New York: Informa Healthcare, 2007. Print.
- Endotoxins and Their Detection With the Limulus Amebocyte Lystate Test, Alan R. Liss, Inc., 150 Fifth Avenue, New York, NY (1982)