In the world of pharmaceutical production, it is universally understood that a robust pharmaceutical quality system provides key elements of assurance and oversight for pharmaceutical manufacturing processes. It ensures that patients are provided with medications that are safe, effective, and reliably produced at a high level of quality. However, despite recent advances in the manufacturing sector, quality issues remain a frequent occurrence, and can result in recalls, withdrawals, or harm to patients. Quality issues have also been linked to the rise in critical drug shortages.¹
Regulatory agencies currently assesses the risk profile of manufacturing sites based primarily on their compliance history, as seen in warning letters and field reports, in conjunction with records on product recalls and market-based quality problems. These are not necessarily the most informative measures, and by their nature, provide historical or lagging data or signal detection. More relevant data relating to the state-of-quality, provided in advance, would better inform the risk factors that might predict quality problems and future drug shortages.
FDA’s approach to quality oversight has evolved in recent years, and the new Office of Pharmaceutical Quality (OPQ) has made it a priority to establish a sounder basis for ensuring that pharmaceutical products meet high quality standards throughout the product lifecycle
This FDA led Research Project initiates a program aimed at developing and implementing a set of standardized manufacturing quality metrics. The establishment and collection of these metrics should provide various stakeholders – from industry to regulators – with greater insight into the state-of-quality at a given manufacturing facility, and allow stakeholders to better anticipate and address quality issues while simultaneously reducing unnecessary regulatory burden.
RECENT DEVELOPMENTS IN THE QUALITY METRICS INITIATIVE
Since early 2013, FDA has been working with the pharmaceutical industry to develop goals and objectives for a metrics program. In response, several industry stakeholder groups have worked with FDA to develop consensus around the goals, as well as identify potential metric sets, including developing recommendations for their implementation and interpretation. Through a series of extensive engagements between industry and FDA there has been an acknowledgement of the complexity of the problem at hand, which is to develop a recommeded set of metrics which are objective and meaningful, easy to capture yet normalized to account for factors such as process differences and technical complexity. Furthermore, it is required that those elements selected will promote acceptable behaviors and not lead to any unintended consquences or unwanted behaviors.
Key Research Questions to Address in the Selection of a Proposed Metrics Set:
1. What is the appropriate set of metrics to collect for establishing the state-of-quality at a manufacturing facility?
2. Will this set of metrics provide adequate information for all types of pharmaceutical manufacturing, such as sterile injectables, bio-pharmaceuticals?
3. How should these metrics be defined?
4. What is the optimal reporting process and what support will be necessary to facilitate timely and uniform reporting.
5. What are the potential unintended consequences which may arise with chosen metrics?
a. Such as measuring OOS rate may result in companies not reporting OOS results or changing their standards of identifying OOS results to minimize the numbers.
b. Review of complaint trending might lead firms to resolve problems superficially without getting to root cause.
6. How can the FDA prevent manipulation of data and its unintended consequences?
7. How does company culture impact the data collection and the metrics?
8. How does the underlying quality culture of a manufacturing facility influence quality performance and how does one correlate its impact through these metrics?
9. How can one represent a set of complex behavior and performance criteria via a set of simple metrics?
10. How can these set of metrics be linked to operating efficiency and performance of the plants? Plants are naturally focused towards improving their profitability and cost. Significant efforts such as six sigma, lean, right-first-time programs are underway across the industry and have deep rooted support in many pharmaceutical operations around the world. Can the reportable quality metrics be a natural offshoot of such improvement programs?
ROLE OF QUALITY METRICS IN RISK-BASED SURVEILLANCE
Quality metrics are widely used throughout the pharmaceutical industry to monitor quality control systems and processes, and many of the components that inform those metrics (e.g., data on process capability output or statistical process control) are already collected and maintained as part of cGMP compliance. Several measures of performance are already common throughout the industry. The challenege is that they are currently defined differently across manufacturers, and even between sites operated by the same manufacturer.
The proposed FDA Quality Metrics program is not the first of its kind; rather, it draws from the example of existing private sector quality improvement programs that collect voluntarily reported, standardized quality metrics from a large and varying array of manufacturing sites, which are then used by participating manufacturers to benchmark their performance against industry standards and their peers.
The collection and analysis of standardized quality metrics can serve several functions:
• At a basic level, metrics should provide a quantitative and objective measure of quality at the manufacturing site, and provide a window at a systems level, to the effectiveness of the oversight and control of operations at a given site.
• Metrics data collection and analysis should also help mitigate or reduce quality related drug shortages and recalls, by allowing for early identification of products at risk of quality failures.
• Metrics provide an opportunity to stratify manufacturing sites according to quality risk and thus prioritize scarce regulatory resources for inspection of plants world-wide.
• Ultimately, these metrics should assist pharmaceutical manufacturers to promote positive behaviors and a corporate culture of responsibility for quality, by providing incentives to improve product and process capability.
Thus, quality metrics may contribute to ongoing broader FDA efforts to reducing risk and improving drug quality.
FDA OPQ OPEX RESEARCH GRANT
The University of St. Gallen is the academic leader today in this effort to establish solid and meaningful OPEX programs around the world. For more than a decade, it has been working hand-in-hand with the pharmaceutical industry globally to assist in developing widely accepted pharmaceutical manufacturing improvement programs. In July, the FDA OPQ granted University of St.Gallen, Switzerland a $468,641 grant to evaluate, enlarge and test the FDA quality metric based on the St.Gallen Operational Excellence database.
St.Gallen will collaborate on this project with Dublin Institute of Technology, Ireland and with Prabir Basu, Consultant and the former Executive Director of the National Institute for Pharmaceutical Technology and Education (NIPTE) in the U.S.A
This St. Gallen research project aims to identify an appropriate set of quality metrics that is integrated with the operation of a manufacturing facility and its underlying quality culture. Over the past 10-15 years the pharmaceutical industry has becoming increasingly aware of and embraced the benefits provided by formal OPEX programs. The initial driving forces behind these OPEX programs were the improvements in operational efficiency and potential cost savings which are often an essential component in the survival for these manufacturing facilities within the competitive global environment. Recent developments in the more mature OPEX programs have shown considerable benefits in the reduction of variation and comensurate improvements in quality through targeting measurable stabilization of the organisational systems responsible for equipment and facilities, quality management, inventory control and mangement oversight.
The significance of the proposed research project is that it will use operational data already available from nearly 300 global plants to establish the metrics, evaluate and validate them. Specifically, since there is extensive buy-in from within the pharmaceutical industry to the existing St. Gallen OPEX program and the benefits from participating in an international benchmarking process, it is believed that industry buy-in for the proposed quality metrics should be easier to achieve. Based on our extensive experience the metrics proposed by the St. Gallen team will also incorporate underlying factors impacting corporate culture such as management commitment and employee empowerment. It is imperative that these underlying factors become an integral part of any meaningful metrics system.
INNOVATION IN QUALITY METRICS
This project builds on a 12 year experience of benchmarking research in the area of pharmaceutical production and management. This database is the one of its kind in the world and is unique in the sense that the data is already widely used by a large number of industry members across the world. The University of St. Gallen has been working together with leading pharmaceutical companies and has great practical and theoretical insights relating to both the current and future challenges in performance measurement for pharmaceutical production.
The evaluation of the proposed metric set will exclusively draw upon the University of St.Gallen OPEX database (no additional data collecting is foreseen). The St.Gallen Global OPEX Database currently consists of more than 300 data sets of pharmaceutical manufacturing sites from plants all over the world, but mainly of US and European based companies. Fig. 1 gives an overview the configuration of the current database:
Fig. 1: Overview of the St.Gallen OPEX database
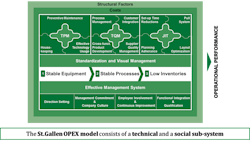
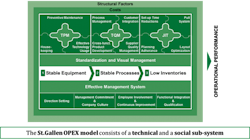
The database includes specific measures of both quantitative KPIs and qualitative Enablers of quality. It combines data from across the technical and social sub-systems within pharmaceutical organizations based on the innovative St.Gallen OPEX model shown in Fig 2.
Fig. 2: The St. Gallen Operational Excellence Model
The technical systems of the St. Gallen OPEX model consists of the elements of:
• Total Productive Maintenance (TPM)
• Total Quality Mangement (TQM)
• Just in Time (JIT)
The first technical system, TPM, consists of measures that are related to the stability of the equipment within a pharmaceutical production facility. Secondly, the TQM measures consider KPIs and Enablers related to the stability of the relevant quality management processes. Lastly, JIT deals with production flow, related inventory levels and lead times. Available data has consistently shown a positive impact of TPM on TQM, and of both TPM and TQM on JIT. Production sites that have a high TPM performance and a high TQM performance have also demonstrated a high JIT performance.
The Social Sub-system of the model addresses a number of management, leadership and continuous improvement elements at a KPI and Enabler level. The benefits of using the formal St. Gallen OPEX model is that it provides opportunities to structure the discussion around quality and operational excellence from a system perspective. This is the fundamental basis for the St. Gallen OPEX database and benchmarking study.
This research proposal aims to relate, adapt and improve the existing OPEX research of the University of St. Gallen to the current needs of the FDA’s quality metrics initiative. The research team will investigate wide known excellence frameworks to detail the evaluations on the basis of existing data. Furthermore, it will use established data analysis methods and tools to show significant impacts and correlations between the underlying qualitative Enablers and the related quantitative KPIs in order to identify patterns and trends. The overall goal of the research team is to keep a system perspective of the entire company environment to capture as many influencing factors as possible and not neglect others. The evaluation will allow for exploring and understanding the interdependencies between Enablers and KPIs from this system perspective.
The outcome of this project will be a verified and proven set of quality metrics. Correlations will not only be tested against traditional quality performance figures like customer complaints or rejected batches but also against overall facility performance and cost figures. Furthermore, the Enablers for quality will be defined based on an overall system understanding taking into account key supply chain elements of supplier quality and distribution performance. Furthermore, the St.Gallen research team adresses the aspect of Quality Culture with the requested improvement culture of the employees.
The next generation quality metrics program should support the aim of achieving enhanced product quality without the need for extensive regulatory oversight and should ultimately help to drive responsibilities for the manufacturers that will lead to a reduction in product-related shortages and quality related recalls. The developed concepts and methods will significantly support the further evaluation and use of the quality metrics to have an influence and positive impact on the industry peers.
1. ISPE. (2013). Report on the ISPE Drug Shortages Survey