Mature Quality Systems: What Pharma Can Learn from Other Industries
Quality has a special meaning in pharmaceuticals, where production or distribution errors can jeopardize human life. But other industries face similar challenges, and some have developed sophisticated quality systems. As pharmaceutical companies look for ways to improve their quality practices and performance, they can take ideas from these quality leaders and adapt them for pharma.
Quality can be defined in various ways, from “fitness for purpose” to “meeting customer expectations” to predictability in statistical terms. Each industry understands quality differently, and priorities depend on the specifics of products and markets. In the automotive industry, for instance, companies generally define quality as the ability to meet (or exceed) customer expectations on products and services. To do so, they work toward Six Sigma levels of high predictability. In addition to avoiding defects, they also strive to maximize the appeal of their vehicles along more subjective criteria such as design, aesthetics, touch and feel, and ease of use.
Aerospace manufacturers, by contrast, still define quality more along the formal requirements of customers and regulators, always ensuring 100 percent airworthiness. They have such complex products that they see quality as an ongoing process of continuous improvement—no airplane was likely ever built right the first time without any deviation or repair. Semiconductor makers focus on design for manufacturability to ensure consistent process quality, as well as to achieve high yield from the precious wafer materials.
Industries with mature quality systems rely on tight protocols and standard operating procedures customized for their respective concerns. They are also known for extensive, systematic, and continuous learning that allows them to address emerging problem areas. Four structural components drive these efforts as a unified “House of Quality”: building quality into the product and into functional processes; organizing for quality in all functional areas and achieving compliance and solid governance; instilling a quality mind-set and behaviors by developing capabilities in everyone, not just the quality organization; and factoring quality into strategy and performance management.[1]
From client surveys and expert interviews, McKinsey has assessed these components in pharmaceuticals as well as three of the strongest industries for quality control: automotive, aerospace, and semiconductor.
PHARMA'S HOUSE OF QUALITY
Quality pressures on pharmaceuticals come mainly from regulatory agencies, which are increasingly driving the industry toward preventive and “intelligent” systems, rather than the control- and audit-based approach sufficient in the past. Quality differences are usually not directly visible to consumers, who are therefore represented by regulators. As a result, Pharma’s quality teams have historically focused heavily on complying with regulatory demands through documentation and testing of samples.
Factories typically show relatively poor quality, with low right-first-time metrics (2 to 3 sigma[2]). But screening by the quality assurance function helps maintain very high quality in terms of the product that reaches customers.
Functional quality processes (built-in quality):
Process quality in Pharma is often fairly low, because processes typically do not translate well from the R&D lab to the reality of the factory floor. Designers are often under pressure to launch quickly, and many do not use quality gates to ensure a scaled-up design for manufacturability. Several issues result:
- Process capability often does not easily meet the design specification, thereby providing a perennial yield issue and an unwelcome deviation-investigation work load. In those cases, production adds secondary processing, such as to remove moisture.
- Factories rarely apply error proofing (poka-yokes[3]) effectively.
- Work standardization is still low, and equipment across sites is different.
- Insufficient validation and verification, combined with overly tight specifications for many things not critical to product quality, create problems that require secondary processing and compensatory testing ,and cause yield loss.
Many companies are streamlining quality control processes, especially in labs, and adopting lean practices, but most have yet to reach best-practice levels.
Quality organization and governance:
Quality groups are independent of production units by regulatory design, and many heads of quality report directly to the CEO or board.[4]Yet many companies still have multiple layers in these groups, leading to cumbersome decision-making. They are also often focused on compliance and documentation, rather than on problem solving, increasing process robustness, and supporting production on demand and in real time.
Quality strategy and KPIs:
Most companies track common production metrics such as first-pass yield, deviations, rejects and rework, compliance failures, recalls, as well as customer complaints. But these metrics are mainly lagging, not leading indicators, and are often followed inconsistently across sites. More important, companies generally lack reliable systems for measuring the true cost of quality and tend to miss or underestimate costs. That leads to decisions on quality investments that are made without understanding either the full cost or the financial value of improving quality.[5]
Quality mind-set, behavior and capability building:
Business leaders, site managers and quality personnel are well aware of quality concerns. But their mind-sets tend to be highly risk-averse. Instead of creatively solving problems, they resist changes and rely on additional checking, oversight, and paperwork-based compliance. Quality culture on the shop floor is still underdeveloped, with quality often seen as the responsibility of the quality organization.[6]
WHERE ARE YOU AT THIS MOMENT?
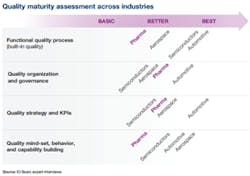
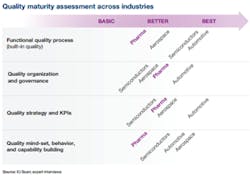
Figure 1.
We can assess the maturity of pharma’s quality efforts in all four of the previously described areas, and compare them with the averages for the three industries with more advanced quality efforts (see Figure 1).CONSUMER-DRIVEN QUALITY IN AUTOMOTIVE
Automobile customers pay close attention to quality and often weigh it equally with price and performance. Quality is much more about product appeal, performance, and reliability than complying with regulation. As their vehicles suffer wear and tear over a period of many years, consumers expect safe and reliable operation, low maintenance costs, and infrequent repairs.
Because of these competitive concerns, automotive companies excel in understanding the voice of the customer. They have become adept at defining a strategy around quality, establishing targets to achieve it, and implementing strong functional quality processes along the value chain—design, development, engineering, production, supplier management, sales, and after-sales. Companies design for reliability over the life cycle so that buyers of second-hand cars have a positive experience as well. With quality a priority for more than 20 years, it has become an entrenched part of management at all levels.
Functional quality processes:
Car companies are particularly strong at embedding quality into operations. They have robust standard work instructions that specify sequencing, work element timings, knack and skill points, and in-process tooling and stock requirements, all with extensive oversight and monitoring that includes visualization. Real-time mechanisms, such as and on cords[7], frequent cadence of reporting, and error proofing (poka-yoke) provide additional feedback, all of which is rigorously used to address issues. A virtual infrastructure of quality, such as a skills confirmation by leaders, supports this control.
In addressing defects in the factory and field, teams follow a structured process with clear schedules for resolution—both the interim containment and the permanent corrective action. They apply the “four eyes” principle for critical processes and usually follow a structured and rigorous problem-solving approach. Toyota, for example, uses an eight-step process supported by standardized A3 reports[8]. Beyond their own operations, automotive companies typically have extensive supplier quality management all the way down the tiers of the supply chain, and sometimes back to the raw materials.
In developing new models, companies take the time to identify issues in previous models and design them out of the new version. They also rely heavily on common design structures, platforming, and common parts. By reusing proven structures, they reduce risk and boost predictability. New-product introductions also strictly follow quality gates and leverage several tools, such as FMEA and QFD, to ensure that products are ready for production.
Challenges remain, especially with the globalization of production. Companies are working to maintain performance levels across geographic locations and suppliers, and even across shifts within factories. Toyota’s goal is to make cars so consistently that all appear the same to consumers regardless of where they were produced.
Quality organization and governance:
Automotive companies typically have strong quality organizations. The head of quality is typically a peer on the top team and reports directly to the CEO. The companies do move back and forth on how much to centralize quality, but this is a balancing act that must change over time. Focusing efforts at the center improves general oversight and drives consistency through policy and common standards, while disseminating quality-related information and lessons learned. Decentralization has the benefit of driving ownership and adaptation to local conditions.
Governance is also disciplined. Periodic reviews take place at multiple levels. Operating teams systematically review all newly appeared defects in the preceding 24 hours. They perform joint “gemba” root-cause analysis and apply countermeasures immediately. Engineering departments discuss outstanding issues at weekly meetings, where they implement root-cause solutions. Executive teams work from a monthly quality board to discuss company strategy and address major actions needed. These structures are usually able to maintain the pace of work while mobilizing the extended organization around quality issues.
Quality strategy and KPIs:
The industry benefits from numerous mechanisms to capture multidimensional feedback on quality, from third-party providers such as JD Power, to company-specific surveys. External sensors, available yearly, are linked to regularly updated and leading internal KPIs, and companies break them down to functional levels with clear accountability. They close the feedback loop and enable continuous performance management. Despite these achievements, automotive companies still strive to understand the voice of the customer better and more quickly. Unprompted feedback in Internet blogs and forums has become a major source of information, especially in important markets like China where third-party providers of customer feedback are not yet well established. Even regulators are becoming more active in this domain. For example, the National Highway and Traffic Safety Association provides customer forums on its Web site for car owners to describe quality problems.
As for understanding the total cost of quality, companies have made a great deal of headway. They tend to focus on the cost from poor quality, so they still lack a full appreciation of the cost of ensuring good quality. But they do regularly use what information they have to make their investments.
Quality mind-set, behavior, and capability building:
Because of all the effort to build quality into the process, however, quality departments have actually been shrinking. Manufacturing engineers, line management, and the general workforce have taken on some of the responsibility not just for post-process inspection but also for proactively improving process capability.
As a result, quality departments are moving away from their old “policeman” role of inspection and playing a more integrated role in the design of the vehicles. They support the product specifications by detailing how to confirm quality. And with more than 60 percent of a car’s value coming from suppliers, much of the quality organization’s focus is now on improving suppliers’ quality systems.
Many companies are still developing their quality culture so that employees and leaders instinctively consider quality in their daily decisions, rather than simply reacting to problems. But their overall success is undeniable: defects in vehicles have fallen by more than 90 percent in the past 20 years, to the point that companies have rescaled their quality metrics to take their vehicles to the next level of reliability.
Safety-Based Quality in Aerospace:
Quality plays out differently in aerospace, where quality failures can be catastrophic. Delivered products are very reliable. But high barriers to entry have allowed companies to pay less attention to reducing the cost of quality. Long product life cycles, combined with stiff regulation, have also meant conservative quality regimes with little change over the decades.
Functional quality processes (built-in quality):
Quality is deeply embedded into manufacturing. Feedback loops to the voice of the customer run from the sales and after-sales areas, following standardized processes. Dedicated task forces are in place to handle improvement requests.
The long product life cycles afford much less opportunity to reflect on quality of design and to learn as in the automotive industry. Instead, companies rely on engineering analytics to ensure the integrity of each component, subsystem, and system, and eventually the plane as an integrated system. All this complexity leads engineers to design in redundancy and safety factors, as well as repair standards for every deviation. For example, if a hole is drilled slightly off from where it should be, the factory will simply increase the hole size to re-center it and use a larger fastener. The net result is high quality, though at a higher cost.
Quality organization and governance:
Stiff regulations have given quality organizations a solid presence in operations. Inspection and quality sign-offs are required for individual jobs throughout the production system. The presence of customer representatives on the manufacturing site, conducting their own quality inspections at multiple points along the line, further reinforces this systemwide commitment to quality. Companies do have opportunities for improvement in coaching people as well as adding financial incentives to quality outcome or quality cost.
Quality strategy and KPIs:
The industry has a solid array of KPIs around quality. The lack of competitive pressures, though, has discouraged attempts to boost transparency and target setting around these KPIs.
Quality mind-set, behavior, and capability building:
Quality mindsets and capabilities are a clear strength of the industry, driven by the high media visibility of quality problems as well as the simple fact that people’s lives are at stake. Companies often communicate their concerns over quality, and they keep employees' quality skill levels generally high.
Yield-Driven Quality in Semiconductors:
In semiconductors, the high cost of fabricating plants and wafer development makes yield the key indicator of quality. The faster a plant can get to producing the wafers, the lower its overall costs. As a result, managers focus on process-level quality. Consumer quality, in terms of specifications and reliability, matters as well but is not a selling point for most of the industry.
Functional quality processes (built-in quality):
Samsung and Intel, which have the fastest (in memory and logic, respectively) ramp-ups to yield, achieve their results with greater investment upfront. They have superior design for manufacturability and for experiment processes, but they also produce before launching more test wafers with full process steps, to identify and fix issues more rapidly. As a result, they reach target yields after only two spins of a new design recipe, while most of the industry will need three or four.
Intel controls for quality with its “Copy Exactly!” strategy, which takes manufacturing standardization to a new level. Because its multiple production sites are essentially identical, they become a single virtual factory matched up to the R&D site. Once the testing factory has a prototype, it rolls the exact recipe to all the factories, with all the same configuration, suppliers, full tools, and processes. Other companies upgrade tools and processes gradually, at lighter upfront cost, but slower ramp-up because of the lack of standardization. All the companies have become very good at technology transfer.
In-process quality controls are also important, so the industry leaders invest more here as well. Process tools for photolithography and deposition are highly sensitive, and parameter control excellence is a prerequisite. The constant feedback loop from the metrology tools used for in-line testing, along with technicians skilled in controlling these parameters and regularly scheduled maintenance, make the high yields possible.
Quality organization and governance:
Fabricating plants have well-staffed quality teams to support the operators. They check and monitor, perform tests, and work with operators on the individual tools.
Quality strategy and KPIs:
Besides yield, companies track uptime and throughput as closely followed proxies for quality. The factory-level numbers are tracked at the executive level of the firm, and yield ramp-ups are usually a company-wide financial objective, tied to bonuses.
Mind-set, behavior, and capability building:
Machine operators are the first line of defense for quality, and they are well trained to detect issues and reroute capacity to a different product until the issue is resolved. Yield is everybody’s priority as the key economic driver of the business, so mind-sets are well aligned.
WHAT PHARMA CAN LEARN FROM OTHER INDUSTRIES
These examples can inspire Pharma in a variety of ways. From the automotive industry, companies can learn to integrate the voice of the customer into the quality system. Not only can they develop external sources of feedback, but they can also translate them into leading internal KPIs. The car industry can also point the way to developing a high-speed problem-solving engine for quality issues. The key is to define a structured and standardized series of meetings and an escalation mechanism in order to secure cross-functional teamwork and share best practices.
Automotive companies are also leading the way in developing strong collaboration with suppliers. Some have built an approach based on fair competition, transparency of performance, mutual trust, and a comprehensive infrastructure to support the suppliers. The assistance ranges from new-product introductions to crisis management and long-term improvement plans for business processes. Some aerospace companies are also making important inroads here.
From semiconductors, pharma can learn the payoff that comes from designing for manufacturability. By front-loading the development process and increasing platforming and standardization, they can improve first-time quality. Experimenting early on can make for better processes during production.
From both semiconductor and automotive companies, pharma can likewise appreciate the importance of moving from an inspection-based regime to an approach with built-in quality. They can standardize the work, design, and production processes with low variability. With strict quality-gate systems and a structured process for lessons learned, an organization can put its efforts into prevention rather than audits.
From aerospace, they can learn the power of a pervasive quality mind-set. Companies treat safety concerns in a proactive way and invest heavily in employee training throughout the organization. Operators do whatever it takes to produce a plane safe to fly.
Pharma face many challenges in moving their organizations toward excellence along all elements of the House of Quality. But they need not be pioneers. They can adapt the structures and practices from other industries to their own specific context.
Editor’s note: This article was originally published in the book "Flawless: From Measuring Failure to Building Quality Robustness in Pharma," available at www. mckinsey.com, under Client service/PMP/Latest thinking"
[1]Andrew Gonce, Lorenzo Positano, Paul Rutten, Vanya Telpis “Flawless: From measuring failure to building quality robustness in pharma,” Flawless: From Measuring Failure to Building Quality Robustness in Pharma, McKinsey & Company, July 2014.
[2]3 sigma=67,000 errors in 1,000,000 opportunities, or 6.7%.
[3] Lean quality mechanisms or techniques that help avoid human error by preventing or correcting mistakes as early as possible
[4]Andrew Gonce, Lorenzo Positano, Vanya Telpis “Beyond the reporting lines: Secrets of successful quality organizations,” Flawless: From Measuring Failure to Building Quality Robustness in Pharma, McKinsey & Company, July 2014.
[5]Katy George, Wolf-Christian Gerstner, Vanya Telpis “Effective quality metrics: The starting point of quality management,” Flawless: From Measuring Failure to Building Quality Robustness in Pharma, McKinsey & Company, July 2014.
[6]Alessandro Delfino, Paul Rutten, Vanya Telpis “The invisible but essential foundation: Fostering a culture of quality in pharma,” Flawless: From Measuring Failure to Building Quality Robustness in Pharma, McKinsey & Company, July 2014.
[7]A system to notify of a quality problem or a process issue. It empowers workers to stop production when a defect is found
[8] An A3 format template including a sequence of boxes to support a structured problem-solving approach