Pfizer Trials Thermal Imaging System For Sealing Integrity Inspection
Thermal imaging inspection is performed through the cap. Upon detection, any defective bottles are removed from the line. The system can measure most bottle and cap sizes and types, and any such transition can be accomplished by choosing the corresponding bottle on the DIR System’s touchscreen menu.
Pfizer Global Supply is set to perform a three-month trial of a packaging integrity testing system based on thermal imaging. In an effort to evaluate the speed, accuracy and cost efficiency of the method in commercial application, Pfizer contracted with DIR technologies to install a 100% inspection system in a pilot trial of the technology designed to investigate an alternative to the destructive seal integrity testing Pfizer currently deploys across a number of packaging operations.PROVING THE CONCEPT
Previous “Proof of Concept” work in Pfizer facilities demonstrated the capability of thermal imaging to potentially enhance the inspection of a number of different packaging operations, particularly the sealing and capping of bottles, as well as sealing operations associated with sterile pouch production.
Steve Hammond, senior director of the process analytical sciences group at Pfizer, explained the company’s interest in this new technology: “Pfizer Global Supply has a long history of deploying new technologies as part of continuous improvement of manufacturing effectiveness programs in support of overall quality. The deployment of thermal imaging technology has the potential to inspect 100% of some packages at normal machine throughput, enabling detection of almost all defects and ensuring the patient only receives a well protected product, while also facilitating the optimization of these operations. The technology to measure many of the key parameters of many package sealing processes has not previously existed.”
Thermal Imaging is an infrared imaging science that detects radiation in the infrared range of the electromagnetic spectrum (roughly 3,000-14,000 nanometers or 3-14 µm), then translates this radiation data into images called thermograms. Because infrared radiation is emitted by all objects above absolute zero, thermal imaging makes it possible to examine an environment without physical contact or visible illumination.
The amount of radiation emitted by an object increases with temperature; therefore, thermal imaging reveals variations or irregularities in temperature. At the height of its precision, thermal imaging can detect changes in temperature so slight that they were previously undetectable or, in the case of commercial ventures, not economically viable until now.
FOIL SEAL INSPECTION
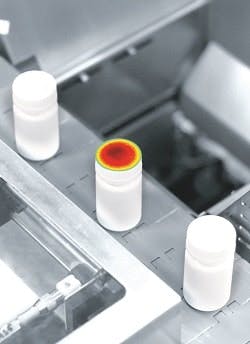
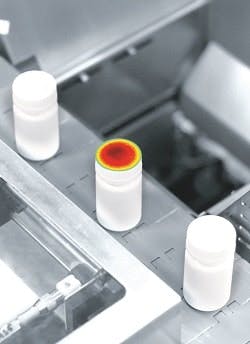
The I2VS (Induction Integrity Verification System) is testing, in-line, the integrity of the aluminum foil sealing of induction-sealed bottles. It is the first system capable of inspecting 100% of the bottles subject to this sealing operation, thus providing Pfizer with continuous process verification without slowing production. At Pfizer, inspection is performed through the cap (because of the technology’s sensitivity). Upon being detected, any defective bottles are removed from the production line. The system can measure most bottle and cap sizes and types, and any such transition can be accomplished by simply choosing the corresponding bottle on the DIR System’s touchscreen menu. The I2VS also hardened for pharma production environments: All external system parts are fabricated from stainless steel or anodized aluminum, and the unit’s infrared camera is sealed against dust and moisture.
DIR Technologies I2VS is testing, in-line, the integrity of the foil sealing of Pfizer’s induction-sealed bottles.
OPERATIONAL PRINCIPLES
Thermal imaging’s operational principles center on the method’s ability to capture the natural thermal radiation emission of all objects with an infrared sensor, and then convert the signals into a 2-D image. The passive nature of thermal imaging makes it ideal for the inspection of pharmaceutical processes, where interaction with the samples under test should be minimized. Thermal Imaging’s precision and non-invasiveness make it ideal for seal verification of plastic bottles, blisters and virtually any kind of flexible packaging including sachets, pouches, stick packs, etc. It can also be used for tablet and liquid fill level inspection, as well as desiccant count/inspection. The technology is portable; units can be wheeled-up and wheeled-away, thus makes them transferable from one packaging line to another in response to operational exigencies.