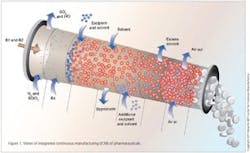
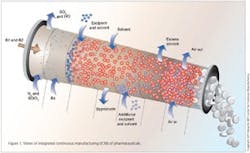
Integrated Continuous Manufacturing
Pharmaceutical manufacturing has been performed using batch technologies for more than a century. While most other manufacturing industries use continuous operations combined with advanced process control and automation, the pharmaceutical industry has mostly relied on fixed recipes to produce batches. This practice results in very long and expensive processes that have been supported by high gross margins. In contrast, the development of novel manufacturing technologies has the potential to transform the current “batch manufacturing” process into a “novel” integrated continuous manufacturing (ICM) process.
This vision for the future of pharmaceutical manufacturing employs the concepts of continuous flow, end-to-end integration, a systems approach, and an integrated control strategy. The vision was initially validated by a team of researchers at the Novartis-MIT Center for Continuous Manufacturing, who designed and constructed a small-scale prototype system to demonstrate that pharmaceuticals can be produced continuously in a fully integrated manner with automated control. In this way, raw materials can be transformed into finished tablets without interruption (24 hours a day), with the active ingredient being synthesized in situ without isolation. This approach opens a new manufacturing paradigm for the pharmaceutical industry with drugs being delivered to patients much quicker, at significantly reduced cost, and with consistently high quality.
BATCH IS INEFFICIENT
Significant advances in science and technology have opened new avenues toward a first-principles understanding of the pharmaceutical manufacturing processes, whose efficiencies lag behind those of many other industries. A decade ago, pharma companies were challenged by U.S. Food and Drug Administration (FDA) Commissioner Mark McClellan who referred to the science of drug manufacturing as being “behind that of potato chips and soap makers.”¹ Unfortunately, there has not been a great deal of improvement so far: The pharmaceutical industry endures losses of ~$50 billion/year in manufacturing costs from inefficient processes.² In many cases, pharmaceutical processes lack advanced on-line quality control systems and rely only on numerous off-line material tests. For this reason, regulators have encouraged the industry to embrace concepts such as Quality by Design (QbD) and the use of advanced process analytical technology (PAT) to produce pharmaceuticals with a higher assurance of acceptable quality at the time and place of manufacture.³
Many of the issues related to the current inefficiency in drug manufacturing originate from the disconnected nature of the processes the industry employs. Current manufacturing practice consists of a series of lengthy and segmented batch process steps often performed in different facilities around the world including isolation, testing, storage and transportation of the various chemical intermediates, as well as the final active pharmaceutical ingredient (API). The API is then transported elsewhere to be formulated into the final dosage form, packaged and shipped to distributors. Because of this disconnect between API and drug product, there is often limited feedback from downstream operators on the desired API’s physico-chemical properties to facilitate its downstream processing. This practice typically leads to a number of “correction steps” that need to be applied to formulate the API into an acceptable dosage form, such as the many APIs that are crystallized as poorly flowable compounds and require micronization and/or granulation before being made into tablets. Also, these series of disconnected steps result in large and expensive inventories.4 Trends in inventory turns are often used as key indicators of improved performance in manufacturing processes and lean management and, unfortunately, in the last decade there have been no significant changes in inventory turns among the top drug companies.5
Batch manufacturing introduces a significant lag-time between technical operations such that the cycle time from the start of manufacture to delivery to patients can be as long as 12 months.6 This practice limits the ability of a manufacturing process to react quickly to changes in demand of a newly launched product or when a large volume of medicine is needed in a relatively short amount of time (such as for flu medicaments). In addition, a recent analysis conducted by the U.S. Government Accountability Office found that the number of critical drug shortages has more than tripled since 2006, especially among cancer drugs and nutritional products, and that these shortages were mainly caused by manufacturing problems, sometimes causing manufacturing shutdowns.7 A recent example of a manufacturing-limited shortage is doxorubicin (Adriamycin), a cancer drug for children; availability of this drug dropped due to manufacturing lacking the capacity to satisfy increased demand.8
The complexity and inefficiency of existing drug manufacturing processes find their roots in the early stages of drug manufacturing process development, where extensive scale-up batches and complex validation procedures are necessary before a new molecular entity can be commercialized, with four major scale-up exercises often being involved (bench, kilo, pilot and commercial). Consequently, clinical trial batches may not be representative of final commercial batches such as for the small molecule drug gabapentin, an anticonvulsant and analgesic, which has been subject to several recalls due to new impurities that appeared after scale-up.9
In such cases when the commercial product does not meet specifications, the entire batch must be rejected, which creates a supply problem. Furthermore, the current scenario for quality control during production, which establishes safety and efficacy of a medicament, is not ideal. Drug makers continue to rely mostly on a ‘Quality by Testing’ (QbT) approach, which involves running a large number of post-processing tests to demonstrate that products meet specifications. Although somewhat reliable, this testing protocol leaves significant opportunities for improvement, as demonstrated by the number of post-approval recalls due to quality issues. This number has not decreased in the last five years (≈ 500/year),10 with the FDA recording an increase of more than 54% compared to average between second and third quarter of 2011.11
NOVEL MANUFACTURING VISION
Although the inefficiency of batch pharmaceutical processes has been well known for decades, the pharmaceutical industry could afford to ignore this inefficiency by focusing their resources on high margin blockbuster drugs. However, the negative forces affecting the pharmaceutical industry today (such as increasing R&D costs, declining new drug approval success rates, major blockbuster drugs going off patent with consequent generic competition, pricing pressure, and introduction of new reimbursement models) make the current manufacturing scenario unsustainable. A transformation of the pharmaceutical development and manufacturing landscape is necessary, and good examples can be found in the oil, gas and food industries that often run their processes in a substantially different way.
In addition, ICM can enable drug manufacturing in smaller facilities with integrated development and manufacturing functions20 and reduced or eliminated product variability associated with scale-up.9 An FDA approved, 20 m2 footprint ICM plant could supply approximately 1.5 tons of drug product per year, which would cover the demand for all clinical trial stages in addition to the launch and early commercialization of a small to medium market medicine. Depending on the overall product demand and related economies of scale, pharmaceutical processes could in some cases be simply scaled-out rather than scaled-up. The new ICM plants will be more flexible and able to react more quickly to market changes due to their compact and small-scale characteristics alongside shorter lead time and on-demand production capability. This approach also has important implications during the launch of a new compound where prediction of the actual demand is rarely good.
Economic analyses reported by Schaberet al.21 on the impact of ICM on the cost of goods sold (COGS) suggest that a small-scale ICM plant will have lower capital and operational expenses by about 30% for the studied case,21 which can be a conservative estimation given that the analysis was based on a bench-scale demonstration unit that was never optimized for commercial manufacturing. Even with this conservative estimate, the global pharmaceutical market has reached approximately $1 trillion by 2013, meaning that roughly $200 billion/year will be spent in the manufacturing of small molecule drugs;22 a 30% savings on the manufacturing of small molecules means that the pharmaceutical industry could potentially reduce its manufacturing costs by about $60 billion/year from transforming their batch operations into ICM.
Finally, the environmental and social impact should be taken into consideration when transformational technologies are introduced into industries with broad societal impact such as pharmaceuticals. We anticipate that integrated continuous manufacturing of pharmaceuticals can reduce waste generation, as reported in many examples of continuous manufacturing,15 and make pharmaceutical processes safer through confined handing of dangerous chemical reactions and high potency materials. Benyahiaet al.23 reported a process environmental factor (E-factor) of 15 for an optimized ICM process with use of recycle, which is at the low end for typical batch manufacturing processes (25–100).24
There are a number of challenges that need to be addressed before ICM can be adopted within the pharmaceutical industry, which can be mainly categorized in terms of three aspects:
1. Organizational/mind set25
2. Regulation
3. Technology
The work published by the MIT researchers addresses the technology aspect in detail, and also provides a novel approach towards control and modeling, which represents important criteria for regulatory approval of ICM processes. Indeed, the transition towards ICM poses new challenges for the control of pharmaceutical processes, and mathematical models can play a crucial role from process development to manufacturing in advancing the different operations and reducing costs.26 One key is the design of an effective control strategy that improves the performance of the plant as a whole instead of individual unit operations. An ICM process is characterized by a number of connected unit operations, which pose challenges on deciding where in the process to intervene to improve the critical quality attributes of the final product.
An integrated control strategy is used to address such questions when developing ICM processes.27 In addition, a dynamic model can be used systematically to develop and test a control structure with feed forward and feedback actions to mitigate the impact of any disturbances to the process in real time, therefore ensuring a consistent high product quality28. Although ICM is mainly characterized by a state of control in its operations, dynamic behavior is observed, including start-up and shut-down, where a model can help to determine optimal conditions to maintain the process in the “acceptable operating range” and therefore minimize waste generation.23, 28
CONCLUSIONS
The pharmaceutical industry is moving into a new era of targeted therapies, where medications need to be developed with specific features and delivered to patients faster. The current batch-to-batch development and manufacturing process, based on the blockbuster model, is costly and inefficient and does not provide the flexibility and quality control necessary for future manufacturing plants. The development of novel manufacturing technologies based on continuous flow can overcome many of these limitations, with Integrated Continuous Manufacturing (ICM)16 being the ultimate goal for this industry.
Derived from a QbD philosophy and based on a first-principles understanding of the process and the application of concepts as continuous flow, end-to-end integration, systems approach, and integrated control strategy, ICM opens a new avenue for developing and manufacturing pharmaceuticals, where active ingredient and drug product manufacturing are integrated into a seamless process. ICM can produce high quality, low cost pharmaceuticals in a few days compared to current lead times of 12 months,6 which results from the segmented nature of the batch plant (including materials holding, shipping, and off-line testing of chemical intermediates and API). These shorter ICM lead times should also alleviate manufacturing-limited shortages.
ICM is being implemented by Novartis in Basel, and more recently by CONTINUUS Pharmaceuticals, a spin-off of the Novartis-MIT Center for Continuous Manufacturing. While science is advancing with the development and fundamental understanding of superior drug manufacturing methods, regulatory agencies and pharmaceutical companies would need to initiate important reorganizations for integrated continuous manufacturing of drugs to be adopted largely within the industry.
ACKNOWLEDGEMENTS
We would like to acknowledge members of the Novartis-MIT Center for Continuous Manufacturing for their valuable inputs on this manuscript. In particular, Patrick Heider, Richard Lakerveld, Haito Zhang, Brahim Benyahia, Paul Barton, Richard Braatz, Charles Cooney, James Evans, Tim Jamison, Klavs Jensen, and Allan Myerson. Finally, we are also grateful to Novartis for its collaborative effort with MIT in developing the novel continuous manufacturing technologies.
REFERENCES
1. Aboud, L.; Henry, S., New prescription for drug makers: update the plants. Wall Street Journal 2003,http://online.wsj.com/article/SB10625358403931000.html.
2. Nickerson, J.; Macher, J., Pharmaceutical industry wastes $50 billion a year due to inefficient manufacturing. McDonough School of
Business, Georgetown University, Washington, DC, and Olin School of Business, Washington University, St. Louis, MO.
2006,http://apps.olin.wustl.edu/faculty/nickerson/results/.
3. (a) Tozer, S. M. Implementation of the new FDA quality by design guidance in pharmaceutical production. MBA thesis
http://dspace.mit.edu/handle/1721.1/44325, 2008;(b) Afnan, A., A time for reflection. Pharmaceutical Manufacturing 2010,9 (5), 25.
4. Shah, N., Pharmaceutical supply chains: key issues and strategies for optimisation. Comput Chem Eng 2004,28, 929-941.
5. Spector, R. E., How lean is pharma. Pharmaceutical Manufactuing 2010,9 (7), 15.
6. Young, S., The future of pharma is incredibly fast. MIT Technology Review 2012,http://www.technologyreview.com/view/427895/the-future-
of-pharma-is-incredibly-fast/http://lgo.mit.edu/conference/speakers/#josephjimenez.
7. Burton, T. M., GAO report blames drug shortages on manufacturing problems. Health Blog, Wall Street Journal
2011,http://blogs.wsj.com/health/2011/12/14/gao-report-blames-drug-shortages-on-manufacturing-problems/.
8. FDA, Current and Resolved Drug Shortages and Discontinuations Reported to FDA. US Food and Drug Administration
2014,http://www.accessdata.fda.gov/scripts/drugshortages/default.cfm.
9. Shanley, A., QbD conference focuses on Gabapentin. Pharmaceutical Manufacturing 2011,10 (7), 16-18.
10. Thomas, P., More drug recalled last year. Pharmaceutical Manufacturing 2010,9 (8), 11.
11. Miller, G., Pharma recalls up 54% in Q3. FiercePharma Manufacturing 2011,
http://www.fiercepharmamanufacturing.com/story/pharma-recalls-54-q3/2011-11-07.
12. Greb, E., The continuous quest for process improvement PharmaTech.com in Equipment and Processing report
2010,http://PharmTech.com/continuousquest.
13. (a) Hartman, R. L.; McMullen, J. P.; Jensen, K. F., Deciding whether to go with the flow: evaluating the merits of flow reactors
for synthesis. Angewandte Chemie International Edition 2011,50, 7502 – 7519;(b) Cybulski, A.; Moulijin, J. A.; Stankiewicz, A., Switching
from batch to continuous processing for fine and intermediate-scale chemicals manufacture. Novel Concepts in Catalysis and Chemical
Reactors: Improving the Efficiency for the Future 2010,Chapter 14, 309-329;(c) Rutter, A., Continuous processing - how do we make it
succeed in the pharmaceutical industry? Royal Society of Chemistry Symposium: Continuous Flow Technology 2011, 15-16.
14. (a) Leuenberger, H., New trends in the production of pharmaceutical granules: batch versus continuous processing. European
Journal of Pharmaceutics and Biopharmaceutics 2001,52, 289-296;(b) Mascia, S.; Seiler, C.; Fitzpatrick, S.; Wilson, D. I., Extrusion-
spheronisation of microcrystalline cellulose pastes using a non-aqueous liquid binder. Int J Pharmaceut 2010,389 (1-2), 1-9.
15. Poechlauer, P.; Manley, J.; Broxterman, R.; Gregertsen, B.; Ridemark, M., Continuous processing in the manufacture of active
pharmaceutical ingredients and finished dosage forms: an industry perspective. Organic Process Research & Development 2012,16 (10),
1586–1590.
16. Mascia, S.; Heider, P. L.; Zhang, H. T.; Lakerveld, R.; Benyahia, B.; Barton, P. I.; Braatz, R. D.; Cooney, C. L.; Evans, J. M. B.;
Jamison, T. F.; Jensen, K. F.; Myerson, A. S.; Trout, B. L., End-to-End Continuous Manufacturing of Pharmaceuticals: Integrated Synthesis,
Purification, and Final Dosage Formation. Angew Chem Int Edit 2013,52(47), 12359-12363.
17. Bharadwaj, S., Pharmaceutical manufacturers set sights on best-in-class operations performance. PharmPro
2008,http://www.pharmpro.com/Articles/2008/01/Pharmaceutical-Manufacturers-Set-Sights-on-Best-in-Class-Operations-Performance/.
18. Muchiri, P.; Pintelon, L., Performance measurement using overall equipment effectiveness (OEE): literature review and practical
application discussion. Int J Prod Res 2008,46 (13), 3517-3535.
19. Heider, P. L.; Born, S. C.; Basak, S.; Benyahia, B.; Lakerveld, R.; Zhang, H. T.; Hogan, R.; Buchbinder, L.; Wolfe, A.; Mascia, S.;
Evans, J. M. B.; Jamison, T. F.; Jensen, K. F., Development of a Multi-Step Synthesis and Workup Sequence for an Integrated, Continuous
Manufacturing Process of a Pharmaceutical. Organic Process Research & Development 2014,18 (3), 402-409.
20. Wilburn, K. R. The business case for continuous manufacturing of pharmaceuticals. MBA thesis, Massachusetts Institute of Technology,
Sloan School of Management, 2010.
21. Schaber, S. D.; Gerogiorgis, D. I.; Ramachandran, R.; Evans, J. M. B.; Barton, P. I.; Trout, B. L., Economic analysis of integrated
continuous and batch pharmaceutical manufacturing: a case study. Ind Eng Chem Res 2011,50 (17), 10083-10092.
22. Basu, P.; Joglekar, G.; Rai, S.; Suresh, P.; Vernon, J., Analysis of manufacturing costs in pharmaceutical companies. Journal of
Pharmaceutical Innovation 2008,3 (3), 30-40.
23. Benyahia, B.; Lakerveld, R.; Barton, P., A plant-wide dynamic model of a continuous pharmaceutical process. Ind Eng Chem Res 2012,51
(47), 15393-15412.
24. Sheldon, R. A., The E factor: fifteen years on. Green Chem 2007,9(12), 1273-1283.
25. Plumb, K., Continuous processing in the pharmaceutical industry: changing the mind set. Chem Eng Res Des 2005,83 (A6), 730-738.
26. (a) Petrides, D.; Koulouris, A.; Lagonicos, P. T., The role of process simulation in pharmaceutical process development and product
commercialization. Pharmaceutical Engineering 2002,22 (1), 1-8;(b) Gernaey, K. V.; Gani, R., A model-based systems approach to
pharmaceutical product-process design and analysis. Chem Eng Sci 2010,65 (21), 5757-5769;(c) Papavasileiou, V.; Koulouris, A.; Siletti,
C.; Petrides, P., The role of process simulation and scheduling tools in the development and commercialization of pharmaceutical products.
Pharmaceutical Engineering 2007,22 (1), 14-22.
27. Lakerveld, R.; Benyahia, B.; Heider, P. L.; Zhang, H. T.; Wolfe, A.; Testa, C. J.; Ogden, S.; Hersey, D. R.; Mascia, S.; Evans, J. M.;
Braatz, R. D.; Barton, P. I. In The Application of an Automated Plant-wide Control Strategy for a Continuous Pharmaceutical Pilot
Plant, Proceedings of the 2014 American Control Conference, Portland, Oregon, USA, Mar; Portland, Oregon, USA, 2014; pp 3512-3517.
28. Lakerveld, R.; Benyahia, B.; Braatz, R. D.; Barton, P. I., Model-Based Design of a Plant-Wide Control Strategy for a Continuous
Pharmaceutical Plant. Aiche J 2013,59 (10), 3671-3685.