Tablet Evaluation Using Ultrasound Transmission Measurement
The mechanical properties of tablets are important because they are related to their therapeutic response. Tablets have to be hard enough for later process stages such as coating and storage. Mechanical failures can originate from the compaction process as well. Known as capping and lamination, these issues can cause tablets to break during coating and packing and cause serious problems during the manufacturing process.
Traditionally, the mechanical properties of tablets have been determined using destructive test methods such as diametrical and bending tests. However, it’s been reported that the diametrical test method has its own limitations, providing an estimation of the crushing force of tablets.1 Because tablet hardness and capping and lamination issues are related to the mechanical properties of tablets, ultrasound (a commonly used test method used to determine the mec hanical properties of test samples in different kinds of non-destructive testing applications) may be a useful tool for the non-destructive evaluation of tablets.
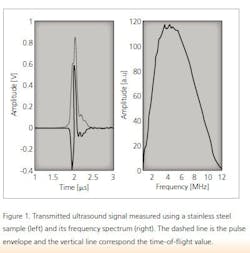
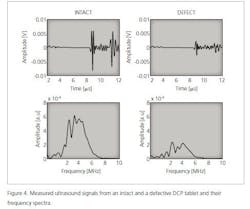
Ultrasound is a mechanical wave with a frequency higher than 20 kHz. In a medium, ultrasound waves propagate at the speed of sound. Ultrasound is generated using a transducer that normally transmits a pulse with a finite length. In addition, the pulse has a nominal center frequency. An example of an ultrasound pulse and its frequency spectrum is shown in Figure 1. An ultrasound pulse is generated via the pulser/receiver unit and the waveform is acquired using an oscilloscope.
The speed of sound of the material under investigation is calculated using the thickness of the sample and a time-of-flight value obtained simply by dividing the sample’s thickness measurement with the time-of-flight value. The time-of-flight value is determined from a measured ultrasound signal and the time that it takes for the wave to travel through a given medium. In this study, time-of-flight was determined using the maximum value of the transmitted ultrasound signal.2 The maximum value of the ultrasound signal is obtained using the pulse envelope calculated using the Hilbert transformation and the corresponding time instant is the time-of-flight value (Figure 1).
During its propagation through a medium, the ultrasound wave will be subject to the energy loss caused by absorption and scattering. The attenuation coefficient is a measure of these losses.3 The ultrasound attenuation coefficient value tells how much the signal attenuates per length, and it is normally given in dB/cm. The frequency-dependent attenuation coefficient “a” is calculated using the equation3:
a(f) =
Where h is the thickness of a tablet, A is the frequency spectrum of the ultrasound signal measured from the tablet; Aref is the frequency spectrum of the ultrasound signal measured from the reference sample (stainless steel in this study). The frequency spectrum of a signal is calculated using the Fourier transformation. In addition, the thickness of the tablet is used in the calculation of the ultrasound attenuation.
TENSILE STRENGTH EVALUATION
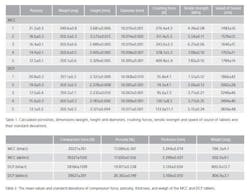
Two commonly used pharmaceutical excipient powders, microcrystalline cellulose (MCC) Avicel PH101 and dibasic calcium phosphate dehydrate (DCP) were used to compress tablets with only one ingredient. The powders were used as received. Before compaction, a helium pycnometer was used to measure the apparent particle density resulting 1.5307 g/cm3 and 2.380 g/cm3 for MCC and DCP, respectively. Tablets were directly compressed using a compaction simulator with 10 mm, flat-faced punches. The compaction profile was a single-sided triangle for the upper punch and a stationary lower punch. Tablet weight was set at 350 mg for all the tablets, but five different compression force values were used to compress test samples to produce tablets with different tensile strengths. Each compression force value had 10 parallel samples, so for both excipient s a total number of 50 tablets were produced.
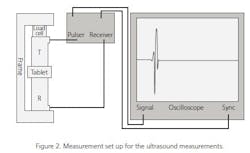
The ultrasound measurements were made using a measurement set-up shown in Figure 2. The speed of sound was measured using a pair of 10-MHz contact ultrasound transducers. The tablet was placed between the transducers and a force of 12 N was applied to ensure proper acoustical coupling between the transducers and the tablet. The speed-of-sound values were calculated using the measured time-of-flight value and the thickness of the tablet. After the ultrasound measurements, the crushing force of the tablet F was determined using a mechanical tester and the tensile strength values were calculated using the equation4
a = 2F/(nDh)
where D and h are the diameter and the height of the tablet, respectively.
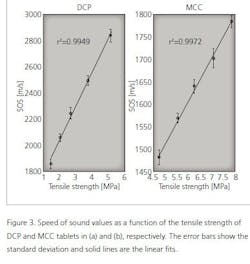
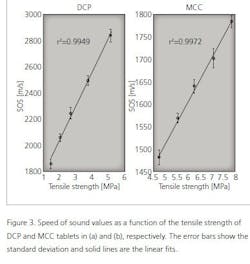
As shown in Table 1, the speed of sound increases with the tensile strength of tablets. Next, statistical tests were made to investigate the relationship between the speed of sound and tensile strength values. The speed of sound values were divided in five groups (see Table 1) and the statistical difference between the groups was tested using the Mann-Whitney U-test. As a result, all groups are statistically different from each other (p<0.05) for both MCC and DCP tablets. To visualize this relationship, the speed of sound is plotted as a function of the tensile strength (Figure 3). As shown in Figure 3, the speed of sound is linearly increasing with the tensile strength. The calculation of the correlation coefficient between the speed of sound and tensile strength yielded the values of r2= 0.9972 and r2= 0.9949 for MCC and DCP, respectively.
However, the high tensile strength value does not indicate the high speed of sound value in general. This is seen when comparing the MCC and DCP groups. The DCP tablets have the higher speed of sound value than MCC tablets, but their tensile strength values are lower than that of MCC. This means that the speed of sound value is suitable for detecting changes in the tensile strength, not the absolute values.
TABLET INTEGRITY TEST USING ULTRASOUND
The same excipient powders as in the previous case are used in this case to compress tablets with only one ingredient. Now there are two set of tablets; one set consists of intact tablets and the other set has tablets with defects. The intact tablets were compressed using the compaction simulator with 13 mm punches (Table 2). The powder masses were 600 mg and 800 mg for MCC and DCP, respectively. The defective tablets were compressed as follows. First, half of the powder was poured into the die, and after that, a small piece of a parchment paper was carefully placed on the top of the powder bed. The size of the paper was 10 mm x 10 mm with the thickness of 92 µm. Next, the rest of the powder was poured over the paper to fill the die. This way, the size of a defect is the same in every defective tablet. The total number of the intact tablets was 6 and 10 for MCC and DCP, respectively, while the number of the defective tablets was 8 and 11 for MCC and DCP, respectively.
The ultrasound attenuation was calculated using the equation1.
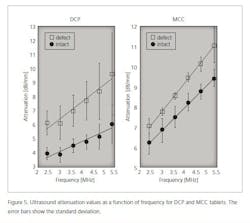
In the case of defective tablets, the amplitude of the ultrasound signal is smaller than in the case of intact tablets. This means that ultrasound attenuates more in defective tablets than in intact tablets (Figure 5).
MEASUREMENTS DURING COMPRESSION
A binary mixture of MCC and paracetamol (PRC) was prepared by mixing of MCC and 30% (w/w) of PRC with a mixer. Before mixing, the powder’s mass was weighed with an analytical balance. The mixing time was 10 minutes at 22 rpm after which the magnesium stearate (MS) was added in the mixture and mixed again for 2 minutes at 22 rpm. Then the mixture was poured in the feed shoe of the tablet press. Ten tablets were compacted with the eccentric single station press using two compression forces (7500 N and 14800 N) and 10-mm instrumented punches. Ultrasound measurements, synchronized with the compaction force and displacement measurements, were made during the compression at an interval of 8 ms.
During compression, the thickness of the powder bed and compression force on the upper and lower punches were measured. The speed of sound i s calculated using the measured thickness. The speed of sound values as a function of time are shown together with the measured compression forces for the upper and lower punches in Figure 6.
The speed of sound increases with the compression force and it reaches the maximum value at the same time as the compression force (Figure 6). After that the speed of sound value decreases until the contact between the tablet and punches is lost. The change of the speed of sound value during the dwell time (Figure 6), the time when the punches are immobile, means that the mechanical properties of the tablet changes and this continues until the signal is lost. It was observed that the force of 3000 N is needed to get a proper ultrasound signal through the powder bed. The end point of the ultrasound measurement was found to be when the upper punch has moved about 0.1 mm up to the minimum value.
This means that the time window for ultrasound measurements is between 440-590 ms. Of course, this will depend on the tablet press and the materials used. The measurement time window and interval are adjustable, making the measurement system flexible and suitable for monitoring the different phases of the compression process.
Ultrasound was found to be sensitive to changes in the mechanical properties of tablets.
The speed of sound value correlates with the tensile strength value of tested tablets. Thus, ultrasound might be used as a non-destructive test method for evaluating possible changes in the tensile strength. Artificial defects inside tablets increased the ultrasound attenuation value compared with the intact ones. Defective and intact tablets can be classified using the measured ultrasound value. Ultrasound measurements can be made during compression by implementing ultrasound transducers inside the upper and lower punches. With the aid of these punches, the speed of sound values can be calculated as a function of the compression time during the t ablet compression.
Published in the October 2013 edition of Pharmaceutical Manufacturing magazine
Author’s Note:
Part of this article was published in 20105 and 20116 in the International Journal of Pharmaceutics.
References
1 Morisseau, K.M., Rhodes, C.T., 1997. Near-infrared spectroscopy as a non-destructive alternative to conventional tablet hardness testing. Pharm. Res. 14, 108-111.
2 Ragozzino, M., 1981. Analysis of the error in measurement of ultrasound speed in tissue due to waveform deformation by frequency-dependent attenuation. Ultrasonics 19, 135–138.
3 Cobbold, R.S.C, 2007. Foundations
of Biomedical Ultrasound, Oxford University Press, New York. Chapter 1.8 pp. 69-87
4 Fell, J.T., Newton, J.M., 1970. Determination of tablet strength by the diametrical compression test, J. Pharm. Sci. 59,
688-691.
5 Leskinen, J.T.T., Simonaho, S.-P., Hakulinen, M., Ketolainen, J., 2010. In-line ultrasound measurement system for detecting tablet integrity, Int. J. Pharm. 400, 104-113.
6 Simonaho S.-P., Takala T.A., Kuosmanen M., Ketolainen J., 2011. Ultrasound transmission measurements for tensile strength evaluation of tablets, Int. J. Pharm. 409, 104-110.
Equipment
Ultrasound pulser-receiver unit:
5077PR, Olympus- NDT Inc., Waltham, MA
Oscilloscope: LeCroy Wavesurfer 42Xs-A (LeCroy Corp., NY
Helium pycnometer: MVP-1: Quantachrome, Syosset, NY
Compaction simulator: PCS-1 Puuman Oy, Kuopio, Finland
Contact ultrasound transducers: Olympus model V112
Mechanical tester: CT-5, Engineering systems, Nottingham, UK
Single-station tablet press: EK-0, Korsch AG, Berlin
Analytical balance: A200S, Sartorius, Goettingen, Germany