The parameters of today’s global pharmaceutical industry, and the highly competitive business arena in which it operates, continue to evolve. Pricing pressures are increasing, generics are moving to the forefront as blockbuster patents expire, and regulatory demands are rising. Driven by an increasing focus on new markets and the development of more targeted medicines, the sheer number of product variants that are being manufactured continues to rise. Although these products are manufactured in lesser numbers than their blockbuster predecessors, market-by-market demand is more highly volatile and increasingly variable.These converging trends challenge companies to adapt their supply chain and manufacturing strategies to the highly competitive and still-evolving demands of new and emerging markets. The risks for not doing so are abundantly clear: higher production costs, greater inventory carrying costs, and potential customer service shortfalls. Put another way, Pfizer’s commitment to provide high quality, competitive pharmaceutical products can only be achieved by continuously working to improve and optimize our performance levels.
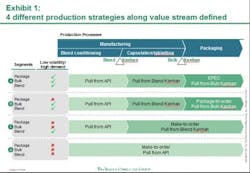
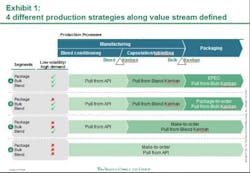
Against this backdrop, Pfizer has laid out a framework to guide the organization on its continuous quest to improve the performance of its supply network. Aligning supply operations with the volatile reality of market demand is essential to delivering these high expectations. Working with the Boston Consulting Group (BCG), Pfizer has developed and implemented a new production strategy at its manufacturing site in Freiburg, Germany. The core of this production strategy: leveraging different supply chain and inventory strategies for different Pfizer products based on their demand and volatility patterns. This segmentation approach—built on a highly flexible pull system with the ability to drive both fixed schedule and fixed volume—tailors Freiburg site production to customer demand in the 180 countries where it sells medicine, and enables next-level optimization. Putting to rest the idea of one-size-fits-all supply chain and manufacturing, this strategic approach reduces the need to maintain high inventory levels to meet any and all possible demand. The result: a flexible, cost-effective system that addresses the challenges of an increasingly complex environment to enable lower inventories and ensure a high level of service.The first phase of implementation focused on the portfolio of one major product, was carried out over six months, and improved the site’s performance on the production costs of these products by approximately 10 percent. The consequently shortened lead times resulted in lower required inventory levels. This initial phase of the new production strategy reduced the total number of changeovers and generally lessened complexity within the production and planning processes. Additional benefits are indicated in the areas of colleague satisfaction and engagement levels. Monthly "pulse checks" (surveys) conducted at the Freiburg site, for example, show increased colleague engagement and willingness to facilitate continued implementation efforts. Pfizer is currently rolling out the new production strategy model to Freiburg and other sites across its global manufacturing networkPfizer FreiburgPart of the PGS network since 2000, Freiburg was already a high-performing site with a long history of driving operational improvement when the new production strategy was implemented. Its 800 Pfizer colleagues produce more than 4.9 billion capsules and tablets and 190 million packs per year. In recent years, Pfizer invested greatly to raise Freiburg’s level of automation. The site initiated lean manufacturing strategies that led to significant improvements in manufacturing, packaging, and quality. Outsiders recognized the results. Freiburg received an industry first “KAIZEN Continuity Award 5S Best in Class 2009 until 2011” from the KAIZEN Institute in 2011. This marked the first time this honor was given to a pharmaceutical manufacturing site. Benchmark studies [e.g. McKinsey POBOS] show that the site ranks highly compared to competitors and multiple Pfizer Customer Service Awards (bestowed in 2007, 2008, 2009, and 2011) have recognized Freiburg's high level of service. Yet Pfizer has taken the Freiburg site to the next level of performance, in part to serve as a model for the implementation of this component of its network performance framework in an already high performing operation. This comprehensive performance framework describes the attributes of “best in class” supply organizations and is designed to help guide the progress towards the PGS Vision and identify opportunities for improvement in day-to-day operations. While Freiburg’s various departments showed marked improvement, the end-to-end processes and the interfaces among departments indicated opportunities for improvement. Furthermore, the site needed to prepare to deal with an expected leap in manufacturing complexity. The number of stock keeping units (SKUs), produced at Freiburg – currently approximately 2,500 -- is forecast to jump by 50 percent over the next five years.The site’s previous production planning process treated all products with a common, central approach that didn’t take different demand patterns into account. Market forecasts triggered production for all products. Because a large number of products shared the same manufacturing equipment thus necessitating lengthy changeovers, this approach resulted in long lead times. The high inventory levels needed to accommodate delivery times raised carrying costs. Moreover, this production schedule was highly inflexible; unforeseen issues in manufacturing required changing downstream schedules in packaging, adding to changeover costs. Workers at times scrambled to "put out fires" caused by urgent supply needs and unplanned events. Planning the New StrategyIt is no secret that maintaining high levels of warehouse inventory makes it easier to deliver high levels of customer service. Pfizer was challenged to gain competitive advantage in a dynamic and increasingly complex business arena by maintaining high service levels and lowering production and inventory costs. In November 2010, PGS and BCG began work on a joint project designed to address this challenge. This collaborative process focused on rethinking how manufacturing complexity was managed and developing more highly efficient Lean production methods; for example, producing a competitive product while at the same time controlling expense and working capital (inventories) as much as possible. The team analyzed Freiburg's high-volume product families, which account for approximately 30 percent of total sales (more than 700 SKUs). Additionally, for each of these products, the team analyzed sales volume and demand volatility and dissected its three main production steps -- blending, encapsulation or tableting, and packaging. This analysis yielded vital data. The same common-form tablet or capsule end product can have vastly different packaging requirements. It is not unusual, for example, for the site to produce a small number of the same common-form capsules for distribution to various markets around the world. However, because each market requires a different package (distinguished by language and often by tablet/capsule count as well), it is not in the manufacturing of the common-form tablet or capsule that the complexity arises, but in its packaging. Analysis identified four different product segments, based on sales volume and demand volatility levels for those different processes. The results, seen below in Exhibit 1, show that 15 percent of the finished goods SKUs, accounting for 75 percent of sales volume, can be identified as low demand volatility, high volume products. The remaining SKUs have either smaller volumes or higher demand volatility—and are mainly products with special dosages or for smaller countries. The team applied two general strategies at different points to determine the optimum production strategy for each of these segments. One of these, the “make-to-forecast” or "push," strategy, says that manufacturing is driven by forecasted demand. According to the "make-to-order" or "pull" strategy, however, manufacturing is triggered by consumption.Aligning Product Strategy to Product SegmentThe different production strategies were aligned into four product segments (see Exhibit 1, below).