Operational Excellence in the QC Lab: Key Issues and an Implementation Approach
By Fred Greulich, Maxiom Group (www.maxiomgroup.com)
Laboratory environments are one of the next frontiers for the application of Operational Excellence (OE). OE applied in Quality Control (QC) labs makes a great deal of sense, allowing organizations to focus on testing that delivers quick, cost-effective results, enable better quality results, provide safer workplaces, and reduce workplace frustration.
Implementing OE principles is also considered by many to be the best way to improve lab efficiency and reduce cost since the principles and techniques have been well proven in many other areas of business, including within the life sciences.
There are, however, a number of unique factors that make OE implementation in life sciences QC labs somewhat different than in manufacturing or other business areas.
For example, there is typically more workload volatility and variability along with less process reliability and predictability (as opposed to manufacturing) and often longer task cycle times. Additionally, in QC labs there is a more frequent need to deal with the abnormal, such as managing out-of-spec results, and there tends to be a mix of routine and non-routine testing along with “non-test” tasks and projects.
As a result, when working with clients to apply OE in QC lab environments, we are often confronted with a “current state” that exhibits some or all of the following behaviors and characteristics. These frequently become the issues around which the work of achieving lab excellence is organized:
- Inefficient labor deployment—resources dedicated by test or task, “weekly bucket” scheduling, or simply mapping available work to the available people;
- Queues and high volumes of Work in Process (WIP);
- Poor processes and significant effort applied to controlling, tracking and prioritizing samples;
- Ineffective “fast track” systems or situations where a significant portion of work is “fast tracked”;
- Lab analyst roles that are not optimized or balanced;
- Lack of defined sequences, batch sizes or standard work;
- Minimal performance management—some focus on individual test accuracy and cycle time, very little attention paid to productivity and efficiency;
- Lack of lab tech cross-skilling and overall poor tech training protocols;
- Long lead times and low productivity;
- Software systems (e.g., LIMS) implemented on top of flawed processes.
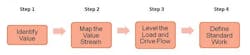
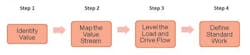
Addressing these factors requires the tailoring of various OE tools and methods, but the basic principles and approach logic remains the same. In QC labs we tend to focus concurrently on developing flow and eliminating waste, utilizing a four step path:
In Step 1, Identify Value, it is important to understand, from the customer’s perspective, the key lab outputs and success measures. This is frequently done through structured one-on-one or small group stakeholder interviews and is sometimes supplemented by a survey, if broader organizational input is desired.
In Step 2, Map the Value Stream, a cross-functional client team develops a map of the entire value stream and subsequently analyzes it in order to identify areas of waste/inefficiency. This step typically includes some team education regarding how to develop the value stream map as well as how to identify the various areas of waste—wasted motion, defects, overproduction, transportation, waiting, inventory, and over processing. Note that in most cases some or all of the behaviors and characteristics outlined previously emerge as key issue areas.
Developing and implementing approaches and work plans to address the identified opportunities also begins in this step.
Very often we recommend beginning implementation by improving lab workplace organization and increasing use of visual management systems, typically utilizing the 5S methodology. Starting here helps in seeing how work moves through the lab, while utilizing visual management boards helps facilitate schedule and issue management.
Step 3, Level the Load and Drive Flow, is critical in QC lab environments and focuses on providing, as much as possible, a level and predictable workload into the lab. In most labs, this issue of fluctuation in overall and individual lab analyst workloads is by far the largest area of waste. Key to this step is identifying a leveling strategy which defines the method to be used for leveling the demand.
In labs, as in other places, a strong link exists between leveling and flow . . . work cannot flow through a lab unless the workload is level, at least on a near-term basis, and fluctuating workloads usually cannot be leveled unless flow is employed.
The simplest and often best leveling strategy is to develop a system to test samples as quickly as possible at a leveled rate of demand. This is done by determining a repeating cycle of testing that allows the samples to move through all required tests quickly. Adopting this strategy reduces the throughput time and allows for holding of samples in a leveling queue at the front end of the process. While in this queue, samples can be sequenced based on customer need. However, when samples enter the lab in the leveled workload, they are run, without exception, in first-in, first-out (FIFO) order.
In Step 4, Define Standard Work, once testing sequences have been identified and other areas of waste and inefficiency have been addressed, standard work needs to be developed, documented, implemented and sustained. This will ensure testing and other lab tasks are completed in the same manner, in the same order, and at the same time in order to be reproducible and predictable and to meet the required demand.
Real Life
While it’s fairly straightforward to understand each step in the process outlined above, real life implementation can be a bit tricky. Design of new “operationally excellent” lab processes is only the first hurdle. A single step move from the old processes to the new one is frequently not possible or, at the very least, not practical. Since manufacturing and other customers must continue to be served, operating under a dual system might be advisable for a period, with gradual phase-out of the old system at the appropriate time.
As with most changes, the temptation to revert to old habits will be tested, but must be avoided. Strong and active leadership is required, as well as frequent communication about the benefits of change.
The move toward applying operational excellence in QC labs is not a one-time project; it is a journey. As with all operational excellence efforts, continuous improvement opportunities for the new processes should be explored and performance managed using appropriate performance indicators.
About the Author
Fred Greulich is Vice President of Operational Excellence at Maxiom Group (www.maxiomgroup.com). He brings extensive skill and experience in the design and implementation of major operational improvement programs at small and large clients. In addition, he has led initiatives at multinational clients in a wide variety of industries including life sciences, food, chemicals & plastics, and consumer packaged goods. He has a B.S. with distinction in Civil Engineering from Worcester Polytechnic Institute. Mr. Greulich can be reached at [email protected].