Managing Pharma Supply Networks in Emerging Markets
For global pharmaceutical manufacturers, emerging economies are becoming increasingly important, both as rapidly growing markets and as sources of supply. Ninety percent of the incremental growth in the global pharmaceuticals market over the next five years is expected to come from emerging economies, for example, while manufacturing sources in these regions already account for 15 percent of the formulated drugs sold in the U.S.
Developing and managing sources of supply in emerging markets is a challenging process for any company, but it is uniquely difficult for pharmaceutical companies, with their highly regulated, quality-focused manufacturing processes and history of vertically integrated production.
Pharma executives frequently ask us about their emerging market supply strategy. How should they select the right supply partners, in a complex and highly fragmented market environment where accurate data on supplier capabilities is not always available? How do they pick the right governance and contract models? How do they manage quality, product safety and delivery risks? How do they ensure their IP is appropriately protected?
Some big companies that have made substantial investment in emerging markets supply are struggling with exactly these issues today. One top-five generics company experienced six months delay in API supply from a large supplier. Another company had to suspend API supply due to poor supplier compliance with technical and quality standards, adding cost and delays to a critical product launch.
For many companies, however, the principal challenges in their emerging market supply networks have been linked to commercial (referring to cost and compensation arrangements with the supplier) and management issues rather than technical ones. Some companies have found that their supply arrangements don’t deliver the cost benefits they are looking for, or that lack of transparency in the relationship makes it difficult to ensure suppliers are complying with quality, cost and delivery performance targets. Others have spent time and effort identifying and engaging many individual suppliers, only to find that these suppliers lack the organizational capabilities to work smoothly together.
Avoiding the External Supply Trap
Having worked with the emerging market supply networks of more than a dozen pharmaceutical companies over the last three years, we have seen that companies can avoid many of the most common issues by doing some smarter thinking upfront. Rather than taking tentative steps and adopting a piecemeal approach in emerging markets, they should think about their emerging market efforts in an integrated way. In particular, we recommend three important departures from today’s common industry practices:
- Plan the end-state of the network first, considering internal capabilities as well as those of potential suppliers.
- Take a more comprehensive approach to supplier qualification and selection, considering cultural issues and commercial as well as technical capabilities.
- Actively manage supplier relationships, with particular emphasis on the first six to twelve months with a new supplier.
Defining the End-state Design
As drug manufacturers build their external supply networks, they can opt for a number of different designs. The network design can be based on straightforward vendor/supplier relationships, either sourcing a small number of products from multiple vendors or a wider range of products from one or two large vendors. It can be built on a collaborative model, with joint ventures, long-term partnerships or technology transfer deals. Alternatively, companies may choose to outsource an entire value chain, with external suppliers handling every stage of production from API manufacturing to packaging and final distribution.
The right model for any company depends on its commercial objectives and its own capabilities. Companies must decide how emerging market capabilities will integrate into their overall networks, for example. Are they willing to totally outsource some types of manufacturing process in order to focus their internal attention on others? Do they have the organizational resources to manage multiple vendors and complex supply chains, or would they be better outsourcing that activity to an organization with more capacity and experience in the region? Do they have an appetite to make investments in assets in emerging markets?
One top manufacturer, for example, has chosen to invest in long-term partnership with two principal suppliers, supplementing any additional requirements with very few, select deals with other suppliers as needed.
Selecting the Right Partners
Once they understand exactly what they need from their supply partners, companies can make smarter supplier selection decisions. While a clear end-state vision will help to focus the search on companies with the right basic qualifications, in our experience there is no shortcut for a comprehensive and time-consuming supplier assessment process, and companies should ensure that this is completed, even if doing so means delaying the start of supply.
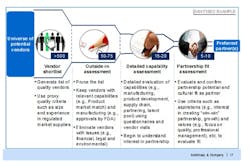
For companies used to sourcing suppliers in developed markets, the lack of available data on their emerging market counterparts can be a shock. Many potential suppliers may be privately, or family owned, making access to even straightforward financial data difficult, let alone accurate information on production capabilities and quality performance. A structured approach (Figure 1) using proxy information—such as the share of overall sales to developed markets as a proxy for quality, or overall size as a proxy for stability—combined with visits and detailed vendor questionnaires is required to build, and then prune, the best possible list of potential suppliers.
Figure 1. A Structured Approach to Vendor Selection
As the characteristics that make or break a successful supplier relationship in the long term are as likely to be commercial as technical, supplier selection should be a truly cross-functional process, with leadership from the top. Is the senior management team happy that it can have a constructive relationship with their counterparts in the supply partner? Are the supply chain, manufacturing and quality functions satisfied that that the partner can provide what they need to make the relationship work?
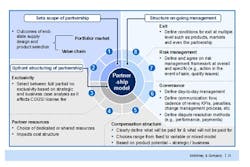
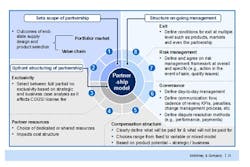
The outcomes of the cross-functional supplier negotiations should also inform the design of any eventual supply contract (Figure 2). Only when a company has a detailed understanding of its suppliers’ capabilities can it determine the best possible allocation of resources between it and its supplier, and put the right risk management and governance clauses in place to ensure the arrangement works as expected. As even the best planned supply relationship can go wrong, contracts must also include an ordered, multiple level exit process to allow the arrangement to be terminated at the product, market or partnership level.
Figure 2. Consider All Aspects of the Supplier Relationship
Actively Manage Supply Relationships
Outsourcing capacity does not mean outsourcing responsibility. In practice, the management of external supply capability requires a different approach to that needed if the same capacity were sourced internally. Even if well-designed contracts stipulate detailed performance criteria and reporting requirements, companies must ensure they have the right mechanism in place to monitor ongoing supplier behavior (for instance, right time delivery, quality) and they should be able to respond quickly to correct issues as they occur. In our experience, this is the step that trips up pharma companies most often, and companies need a proactive approach to the management of new supply contracts. Another “Top Ten” pharmaceutical company placed a supply executive at its partner to do this. Another scheduled monthly supplier reviews with senior management at the beginning of the relationship.
In previous publications [1,2], we emphasized the importance of establishing rigorous procedures for performance management and issue resolution as well as designing an effective organization to support the external supply network. The organization size and nature, understandably, will vary depending on the size, complexity and strategic importance of the deal. At minimum, this can be an account manager within the purchasing function, but such a light approach should be limited to the very simplest, non-strategic supply arrangements. Most substantial ventures require a dedicated cross-functional management team, either based at corporate HQ or, ideally, on the ground in emerging markets where staff can build strong relationships with their counterparts at the supplier and respond quickly when things go wrong.
In most cases, there will be a handover between the cross-functional team that negotiated and established the new supply relationship and the one the will run it. Companies must manage this process with great care, particularly as it happens during the early stages of supply, when problems and disputes are common as company and supplier iron out their working relationship. It is vital that the resources are in place at the company, whether in the transition team or the supply management organization, to proactively manage this situation, insisting on thorough root cause analysis of any quality and delivery problems, for example, and calling the supplier to task on late or missing management information.
The cost, capacity and market-access benefits of emerging market supply networks will be critically important to most large pharmaceutical manufacturers over the next decade. The performance of those global networks tomorrow is rooted in the decisions companies make at home today.
About the Authors
Vikas Bhadoria and Jaidev Rajpal are leaders in McKinsey & Company’s Pharmaceutical Operations practice
References
1. Phillips, R., and Sachs, K. Meeting the Supply Management Challenge. Pharmaceutical Manufacturing, May 2011.
2. Dedeurwaerder, T., Paulson, C., Phillips, R. Mastering the Supply Management Challenge. McKinsey Operations Extranet, June 2010.