Editor’s Note: For a broad overview of BioPlan’s Survey, read “Biopharma Faces the Future.”
Disposable process equipment is on its way to becoming an industry standard, with 30% demand growth projected over the past few years [1]. Stepwise acceptance of single-use disposable components has allowed the technology to progress from simple fluid handling applications involving storage bags and tubing to complex bioprocessing.
Today, we see a growing number of commercially viable, up- and down-scalable bioreactors being developed, and we will likely see fully integrated, up- and downstream disposable technologies being adopted in the not-too-distant future.
At this point, though, disposable systems are not fully accepted in the industry. BioPlan’s sixth and latest Annual Report and Survey of Biopharmaceutical Manufacturing (see Pharmaceutical Manufacturing, April 2009, p. 16), examined disposables along with nine other critical areas associated with biopharmaceutical production, eliciting responses from 446 production executives at drug developers and CMOs in 35 countries. Survey results identify key user technical concerns as:
- Leachables and extractables
- Scale of operation for bioreactors
- Costs of chromatography
Regulatory concerns about L&E have diminished slightly. Where 17% of respondents considered this a top issue last year, only 10% did this year (Figure 1). Similarly, “lack of disposable equipment that meets process requirements,” the primary user concern last year, has drifted into a lower priority status.
Budget Concerns Increasingly Critical
In fact, in this year’s survey, three of the top four issues were cost-related, rather than technical. The top concern, “We have already invested in equipment,” suggests that decision makers are not convinced that, for their applications, they need to change to disposable systems.
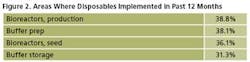
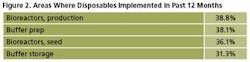
Single-use products are being adopted in new areas of biomanufacturing at an increasing rate, especially for the more technically advanced disposables. For example, production bioreactors were newly introduced to 39% of respondents’ facilities over the past 12 months (Figure 2).
Reasons for Increasing Use
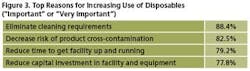
We identified 25 reasons that respondents considered to be factors in deciding on the use of single-use technologies. This year, the top reason continues to be to “Eliminate cleaning requirements,” noted by 88% as being “Important” or “Very important.” Following were “Decrease risk of product cross-contamination.” Then, “Reduce time to get facility up and running” (79% this year, 74% last), and “Reduce capital investment in facility & equipment” (78% this year, 73% last year). (See Figure 3.)
What’s Holding Disposables Adoption Back?
We identified 26 possible reasons that manufacturers may be restricting their use of disposables. The primary reason biopharmaceutical manufacturers and CMOs might not expand their use of disposables this year, as in previous years, was concern over L&E. This year a total of 64.2% of respondents “Agree” or “Strongly agree” that L&E issues are restricting their use (Figure 4).
This compares with 75.4% of respondents last year. Part of this is likely the result of the increased use of disposables, which has, in turn, increased awareness and regulators’ interest.
The second greatest concern this year regarding the use of disposables was “Breakage of bags and loss of production materials” (which grew to 62.5% from 54.9% last year). Following these factors were concerns about cost of disposables (60.8%), and “Becoming vendor-dependent (single-source issues),” and “Material incompatibility with process fluids.” (51.1%)
The top three factors cited this year:
- Leachables and extractables are a concern (cited by 64.2% of our respondents)
- Breakage of bags and loss of production material is a concern (62.5%)
- High cost of disposables (consumables) (60.8%)
Pointing out the potential risk of E&L to patients during clinical trials, Ingrid Markovic, PhD, an Expert Review Scientist for FDA CDER, has emphasized the need to develop appropriate detection and continuous monitoring systems for these compounds and to establish acceptable limits [1]. She suggests that every biologic protein product and its accompanying packaging system be evaluated on an individual basis using common scientific principles.
Who Should Perform L&E Analysis?
|
Survey Methodology |
Testing disposable devices for leachable and extractable contaminants in biomanufacturing requires a clear process, given the infinite variety of conditions such as contact times, pH, and so on. We found that 82% of our respondents agree that “vendors should generate L&E data, and validate.” This suggests an opportunity to vendors. And although 47% indicated that they expected to generate their own L&E data “for Phase III or commercial applications,” fully 22% indicated that they would pay 25% more for disposables if they came with useful vendor-generated L&E data (in fact, 9% would pay 50% more!).
“The issue of leachables and extractables will remain and will need to be handled on a product by product basis given the influence that the particular process solutions have on the extent of leachables/extractables,” says Robert S. Tenerowicz, Vice President of U.S. Operations for XOMA LLC. Tenerowicz believes that the more that vendors can work collaboratively with customers and regulatory agencies to assure the safety of products manufactured with greater product contact with disposables, the easier will be regulatory approvals for all users.
Breakage of Bags
While the bag breakage issue is no longer a critical issue to biomanufacturers, it was still cited by 63% of our respondents as a reason they might not increase usage. This is up from 55% last year, and up sharply from the previous two years’ surveys (44% for 2006 and 35% for 2005).
Cost of Disposables
The cost issues were a disproportionately greater concern among CMO respondents, where 70% agreed that cost of disposables (consumables) was a reason for restricting disposables usage. This compares with 59% of biotherapeutic developers.
Single Vendor Dependence
Another factor that could significantly limit the use of higher-end, unique or proprietary disposables is a fear of becoming too dependent on a single vendor (52% indicating this was a concern). While there are ways to avoid this, many biologics manufacturers refuse to put their commercial production capacity at risk if a vendor becomes unable to supply key manufacturing components.
Scalability of Disposables to Commercial Applications
In most cases, biomanufacturers want to ensure they can scale up, and scale down their processes. As such, switching from disposables to fixed (stainless steel) systems in late-stage manufacturing has historically been something to be avoided. Further, most companies want to avoid designing a disposable process step that would have to be changed for commercial production in stainless steel. This can mean regulatory issues, delays and more costs. The further down the product development pipeline toward commercialization, the more carefully companies consider the impact of switching their manufacturing methods.
We surveyed respondents to determine how likely they would be to start with a disposable production system, knowing they would be switching to a stainless/fixed system prior to full-scale commercial production. Our survey posed the following prompt: “I would use major disposable components in late-stage clinical production even if I expected to switch to fixed/stainless steel systems at the commercial production scale.” This year we found 38.3% “Agreed” or “Strongly agreed” that they would use disposables at intermediate manufacturing scales. This suggests that over a third of biomanufacturers would continue to use disposables even if they recognized they would be moving to stainless steel, fixed systems at the commercial scale. In comparison, last year 46.8% stated the same.
The percentage of respondents willing to change from disposables to fixed production toward late-stage manufacturing appears to be declining relatively quickly. It is possible that the 38.3% who are not concerned about the prospect of switching at late stages are either CMOs, or are very early in the development stage and have not planned for late-stage production, or are expecting it is possible that disposables can be usable at larger, commercial scales. It also can be related to the level of regulatory inexperience in process development.
“The question of switching from disposable to fixed/stainless steel system for late clinical to commercial scale production may also be related to the extent of process characterization that companies typically perform prior to late-stage clinical production,” says Abdul Wajid, Ph.D., Senior Director, Process Sciences, at XOMA. “If companies understand the min-max of each operating parameter sufficiently enough, they may be more willing to make a change later in development since they will have confidence that they can monitor any variations in processing from making the switch. . . . The more process characterization done earlier in development the less likely there may be regulatory delays in making the switch.”
Downstream Issues
Because of the high cost, and good performance of current chromatography resin technology, these technologies are not quick to go disposable, and a disposable, single-use alternative will need to cost less, be at least as effective, and be able to pass regulatory scrutiny.
Single Most Critical Reasons for Restricting Use of Disposables
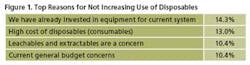
When asked to indicate the most critical reason for not increasing the use disposable technologies this year, 14.3% of respondents said “We have already invested in equipment for current system.” This compares with 18.9% in last year’s study. Second on the list this year was “High cost of disposables.” This is not unexpected in the current budget situation. Following was “Leachables and extractables are a concern”
(with 10.4% vs 16.6% last year) and “Current general budget constraints,” which tied for third (10.4%).
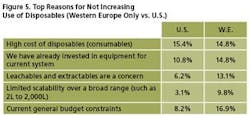
Reasons for Not Increasing: U.S. vs. Europe
We evaluated the single most important reason that respondents might not increase their use of disposable technologies, based on geographic location: U.S. vs. Western Europe (Figure 5). Leachables and extractables were of significantly greater concern to Western European respondents, while general budgetary concerns were more pressing to U.S. respondents.
Reasons cited more often by Western European respondents than by U.S. respondents:
- Leachables and extractables are a concern (13.1% of Western Europeans vs. 6.2% of U.S. respondents)
- Limited scalability over a broad range (e.g., 2 to 2,000 L) (9.8% vs. 3.1%)
- Already invested in equipment for current system (14.8% vs. 10.8%)
- Don’t want to become vendor dependent (6.6% vs. 3.1%)
Reasons cited with much greater frequency by US respondents:
- Current general budget constraints (16.9% of U.S. respondents vs. 8.2% of Western Europeans)
- Regulatory reasons (9.2% vs. 6.6%)
Sources have noted that the European market is growing faster than the U.S. for these disposable products. As far as availability of product, there is no difference between U.S. and Europe. Regarding the response “For regulatory reasons cannot switch,” Europe tends to be stricter in terms of materials requirements, especially when animal-derived components are concerned.
Reasons for Not Increasing: Biotherapeutic Developer vs CMO
We found some significant differences when comparing therapeutic developers’ single most important reason for restricting disposables with CMO’s. The most apparent difference was budgetary, where CMO’s cited “General budget constraints” more than twice as often. Interestingly, biotherapeutic developers found the “High cost of disposables” to be the most objectionable factor, twice as often as CMO’s.
Cited more often by therapeutic developers:
- High cost of disposables (consumables) (14.3% of developers vs. 7.1% of CMO’s)
- No scientific evidence exists to justify the change (6.3% of developers vs. 0% of CMO’s)
Cited more often by CMO’s:
- General budget constraints (17.9% of CMO’s vs 8.7% of developers)
- Do not want to become vendor dependent (7.1% of CMO’s vs. 4.0% of developers)
Waste Disposal of Single-use Devices
The industry is expressing an increasing awareness and concern over environmental and disposal issues associated with disposable products. In this year’s study, for example, more than 23% of users indicated that waste disposal issues either “Significantly” or “Very significantly” reduce their usage of single-use disposables (Figure 6).
Although single-use devices can be challenging to dispose of, they nonetheless represent a good compromise by reducing requirements for cleaning (less energy consumption and time, and less waste water treatment required before discharging in the environment) while ensuring high product quality and optimization of capacity utilization. At low levels of usage, it is not a critical issue, but could be significant in the future.
U.S. decision-makers may be more sensitive than Western Europeans to waste-disposal issues. While 6.3% of U.S. respondents indicated that waste disposal issues “Very significantly reduce usage,” none of their European counterparts indicated the same. Of our U.S. respondents, only 51% claimed that disposal issues do not affect their usage, compared to 60% of Western European respondents.
Conclusion
As disposables become more widely used, decision-makers are more familiar with the details of that usage, are more informed about single-use products, and have established more specific opinions regarding implementation in their unique situations. With more disposable projects in clinical development and commercial production, design engineers and drug innovators are developing more innovative ways to implement this technology. However, until the regulatory hurdles are crossed, issues such as leachables and extractables, alternatives to column chromatography, and the uncertainty of the regulatory process will continue to block adoption of disposables as a more universal, cost-effective solution to bioproduction.
About the Author
Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in 1989, and located in Rockville, MD. For more information, visit www.bioplanassociates.com.
References
1. Langer E, Ed., 6th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, April 2009, BioPlan Associates, Inc. Rockville, MD.