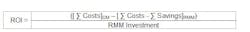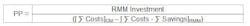The implementation of rapid microbiological methods (RMMs) has been gaining momentum for years. For the most part, the pharmaceutical industry has acknowledged that regulatory agencies are accepting of RMMs and encourage their use, and that PDA Technical Report #33, USP <1223> and EP 5.1.6 all provide sufficient guidance on validation strategies.
Although the industry has the necessary tools for putting alternative microbiology technologies in place, a perception that the long-term benefits will not outweigh the short-term costs persists among manufacturing site heads and senior management teams. For many companies, this has resulted in a significant delay or abandonment of meaningful RMM implementation plans. Therefore, it is imperative that the industry understands how to develop a comprehensive business case and economic analysis of the proposed RMM, and link this information to the long-term technical benefits that the new method will provide.
This paper will provide an overview of the financial components that should go into an RMM business strategy, and practical examples of how to calculate the return on investment and payback period for a real-time RMM.
The Perception
It is understood that the costs associated with the purchase, installation, qualification, and implementation of RMMs can be significant. This should not be a surprise, as most new manufacturing technologies, including those used for PAT applications, require an initial upfront investment in time and expense in order to successfully commission the technology and put it in place for routine use. Unfortunately, many senior leaders in our industry dismiss the potential long-term benefits and only “see” the upfront expenses, such as the capital cost, the validation and calibration fees, the maintenance contracts and headcount dollars required to conduct the validation studies and installation activities. Furthermore, as soon as we bring in the microbiology perspective and start talking about changing the manner in which results will be reported and the century-old colony-forming unit (CFU) becoming a thing of the past, the communication process shuts down, and the word “rapid” is used only to demonstrate how quickly we end up cancelling an exceptional RMM idea.
It is this author’s view that the industry is missing out on a great opportunity for moving microbiology out of the laboratory and onto the manufacturing floor, as several RMM technologies now offer real-time or close to real-time results, single-cell detection, enhanced accuracy and precision, higher throughput and superior data handling and trend analysis capabilities. If we are to tear down the RMM implementation barrier and remove the financial fears associated with implementing these new technologies, it is necessary to fully understand both the costs associated with the initial investment as well as the longer-term financial benefits. We can do this by developing a comprehensive economic analysis that will clearly demonstrate that the long-term benefits far outweigh the short-term expenses.
Return on Investment (ROI) and Payback Period (PP)
ROI is the ratio of money gained or lost on an investment relative to the amount of money invested. For RMMs, the cost of performing the conventional method (CM) with the cost (and savings) of using the new method can be compared. The resulting data is reported as a percentage (%) and usually represents an annual or annualized rate of return. The ROI is calculated using the following formula:
The PP is the time required for the return on an investment to "repay" the sum of the original investment. In the context of RMMs, this equals the time (usually in years) required to realize sufficient cost savings to pay for the initial investment of the RMM capital equipment, qualification and implementation activities. The formula used to calculate the PP is the inverse of the ROI formula:
In order to effectively use the ROI and PP models to economically justify moving forward with an RMM purchase, validation, and implementation plan, it is necessary to gather the appropriate information that will go into the models’ formulas. This information will include the operating costs for both the proposed RMM and the current method (e.g., cost per test, cost of labor, depreciation, overhead, preventive maintenance), the RMM investment (e.g., capital purchases, validation costs, training) and the cost savings or cost avoidances realized with the RMM (e.g., reduced testing time and testing costs for product release, a reduction or elimination of off-line assays, laboratory overhead, resources and equipment, lower cost of product sold, decreased re-sampling, retests and deviations, reduction in rework, reprocessing and lot rejections, and a reduction in plant downtime).
There may also be some regulatory filing costs associated with the RMM; however, if the existing microbiology method is an in-process test or is not specified in an NDA or marketing authorization, a formal regulatory submission to implement the change may not be required. Monetary values for each cost component can be entered into the ROI and PP formulas. The ROI can be calculated for the first year (when the initial capital investment will be made) and then every year thereafter. The rate of return can take on any value greater than or equal to -100%; a positive value represents an investment gain, a negative value represents a loss, and a value of 0% corresponds to no change. The higher the ROI value is, the greater the return will be on the initial investment. Finally, when the information entered for the PP is based on annual values, the PP result will be reported in years.
Case Study: Justifying RMM for Environmental Monitoring
To illustrate how ROI and PP can be used to justify the implementation of a new RMM, one pharmaceutical company investigated ways in which it could employ an alternative microbiology method for environmental monitoring (EM). The company determined that active air sampling was the most labor-intensive and expensive component in its EM program, and identified an optical spectroscopic RMM, the BioVigilant IMD-A, as a potential replacement for its existing agar-based bioaerosol sampling procedure. Because the IMD-A continuously monitors both viable and nonviable particles in real-time, eliminates manual sampling and laboratory testing, and does not use consumables or media, the company presumed it could realize significant cost savings by implementing this RMM.
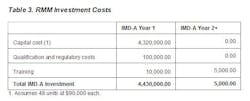
The company gathered monetary values for each operating cost and cost savings component for use in ROI and PP calculations, and applied these calculations to one of its aseptic manufacturing facilities. The facility processes 100,000 air samples per year. Manufacturing is peformed in conventional cleanrooms and the process is personnnel intensive, resulting in the rejection of three $500K product lots per year due to EM excursions on conventional media. This site also shuts down a line three time per year to conduct EM investigations. Tables 1-3 provide detailed information about the financial components that will be used in the ROI and PP models.
Table 1. Operating Costs for Conventional Methods vs. RMM
- Because the IMD-A operates continuously, in this example we will assume that the actual number of tests performed can be reduced by a factor of 5 as compared with the CM.
- Depreciation for IMD-A equals 10% of capital cost (assumes 48 units at $90,000 each; pricing used is representative and is for calculation purposes only, as the supplier may vary the price based on configuration and quantities purchased).
- Annual maintenance and service contracts start in year 2 and are based on geographic region and services contracted. Pricing assumed equals 12% of capital cost (48 units at $90,000 each).
ROI and PP Results
The manufacturer calculated the ROI and the cost savings for the first year, the second and subsequent years, and the total ROI and cost savings for a five-year period. The PP was also determined. A summary of these results is provided below.
The ROI and PP data generated for this facility clearly demonstrate sufficient economic justification for initiating a qualification and implementation plan using the chosen RMM as an alternative method to conventional active air sampling. Additionally, the PP is relatively short due to a substantial cost savings during the first year of implementation. From a business perspective, the use of this RMM would directly impact the company’s bottom line and should satisfy the financial expectations of site management.
The ROI models presented here can be applied to any RMM, although there will be different line items for each technology, such as cost per test, capital cost, maintenance agreements, labor time and laboratory overhead. With the BioVigilant system, because there are no consumable costs, overall cost savings will be greater than for most or all RMMs currently available. Nevertheless, whatever the RMM, calculations such as these will go a long way towards clarifying ROI and therefore justifying investment in these technologies.
Summary
When developing a strategy for introducing RMMs in the manufacturing environment, the use of financial models, such as ROI and PP, can play an important role in providing the necessary justification for purchasing the capital equipment and initiating the qualification program. A robust economic assessment coupled with a comprehensive implementation plan is the key to successful RMM installations and the continuous improvement of manufacturing processes and operational efficiencies.
About the Author
Dr. Michael J. Miller is President of Microbiology Consultants, LLC (http://microbiologyconsultants.com). He is an internationally recognized microbiologist and subject matter expert in pharmaceutical microbiology, Process Analytical Technology (PAT), isolator design and qualification, and the due diligence, validation, registration and implementation of rapid microbiological methods. Over the past 20 years, Dr. Miller has held numerous R&D, manufacturing, quality, consulting and business development leadership roles at Johnson & Johnson, Eli Lilly and Company, Bausch & Lomb, and Pharmaceutical Systems, Inc.
Dr. Miller has authored over 90 technical publications and presentations in the areas of rapid microbiological methods, PAT, ophthalmics, disinfection and sterilization, and is the editor of PDA’s Encyclopedia of Rapid Microbiological Methods. He currently serves on a number of PDA’s program and publication committees and advisory boards, and is co-chairing the revision of PDA Technical Report #33: Evaluation, Validation and Implementation of New Microbiological Testing Methods.
Dr. Miller may be reached at [email protected].