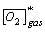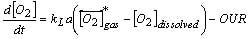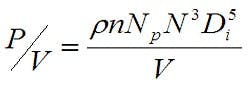Bioreactor Operational Excellence: Best Practices from Scale-up to Control
The modern cell culture bioprocess has been successfully scaled up to volumes greater than 25,000L through sound engineering fundamentals and thorough process understanding. This hasn’t happened by accident, but rather by bioprocessing professionals taking a systematic approach to characterizing the bioreactor’s capabilities and tendencies, developing robust and reliable scale-up procedures, and establishing and maintaining proper control criteria. Those manufacturers that identify and document operational best practices for a cGMP cell culture plant also tend to be those that sustain operational success and deliver high-quality biopharmaceuticals to patients in a timely and reliable manner.
Identifying and documenting bioreactor operation best practices allows for more robust processing by helping to properly educate the operations, engineering and technical staff who oversee the bioreactor processes. Shared learning helps to reduce the amount of “tribal knowledge” that exists within a group and to maintain high levels of operational excellence even in times of employee turnover, with the end result being a sustainable and reliable supply of biopharmaceuticals.
With these ideas in mind, we have set out to document best practices that we have learned for bioprocessing, most notably in the areas of equipment design and overall process control.
Bioreactor Design
Starting off with the proper bioreactor design can resolve many process issues before they arise. One key bioreactor design issue that should not be underestimated is the importance of geometric similarity between bioreactors: maintaining aspect ratios, impeller sizing ratios, impeller spacing ratios and baffle size and location will greatly increase the probability of success at scale. A properly designed bioreactor can lead to reduced qualification and process validation timeframes, as well as increased apparent process robustness and operational success.
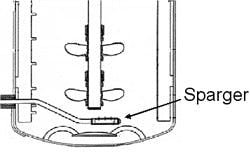
Another key aspect to bioprocess scale-up success is the design of the sparger. Often, two spargers are installed in the production bioreactors while only one sparger is used for the seed bioreactors. While various types of spargers have been utilized within the industry, we have successfully implemented the use of drilled pipes and sparge stones. The drilled pipe yields large bubbles and a lower kLa (which will be discussed below), while the sparge stone yields small bubbles and a very high kLa such that a greater amount of oxygen can be delivered into the cell broth for the same gas flow rate. Figures 1 and 2 illustrate the sparger location and two sparger types.
(Click to enlarge image) Figure 1. Illustration of the sparger location within a bioreactor (not drawn to scale).
(Click to enlarge image) Figure 2. Comparison of bubble sizes erupting from sparge stone and drilled pipe (reproduced from www.mottcorp.com).
The kLa can be mapped as a power law function of the power/volume and superficial gas velocity. Understanding of the kLa allows for estimation of the OUR capacity of the bioreactor, prediction of required oxygen flow rates and prediction of the time-course profile of the dissolved carbon dioxide levels.
Based on process needs and sparger capabilities, the process engineer must determine the preferred configuration for the bioreactors. If process OUR needs are sufficiently low, the default configuration can be the use of one drilled pipe in the seed bioreactors, and two drilled pipes in the production bioreactors. The combination of a drilled pipe and sparge stone may also be used, but the oxygen transfer ability afforded by the sparge stone is typically not necessary. Use of the sparge stone should be avoided if possible due to the increased operational complexity associated with bioreactor set-up, manual changes in gas flows during a process to maintain dissolved carbon dioxide levels, increased foaming and potential cleaning concerns.
Mixing Characterization
When scaling up a free suspension cell culture bioreactor, a thorough understanding of the mixing characteristics is essential. If the mixing inside the bioreactor is appropriately controlled, then the cells will experience an environment very similar to that of the bench-scale bioreactor and will therefore be much more likely to behave as they did in the scale-down bioreactors.
The literature shows that many methods of scale-up have been considered, including matching power/volume, impeller blade tip speeds, bulk mixing Reynolds numbers and bulk mixing times. Due to the nature of these various parameters, it is not possible to maintain them all during scale-up under one set of conditions.
Experience has shown that maintaining a similar power/volume (P/V) at the various bioreactor sizes greatly increases the probability that the mixing within the bioreactor will be appropriate. P/V is a function of the impeller geometry, the agitation rate and working volume, as shown in Equation 2, where ρ is the density, n is the number of impellers, Np is the impeller type power number, N is the agitation rate, Di is the impeller diameter and V is the liquid volume.
To further characterize the mixing, the bulk mixing time at various agitation rates can be measured via pH or conductivity, or calculated using commercially available models. This is performed to determine the length of time required for the bulk liquid to become 99% homogeneous with respect to pH or conductivity.
Combining the results of the kLa mapping, mixing time determination and P/V calculations can lead the process engineer to choose the appropriate agitation setpoint to enable successful scale-up. Once the agitation setpoint is determined, the sparging scheme can be designed to ensure the dissolved oxygen in the bioreactor is maintained while carbon dioxide is effectively stripped from the bioreactor and foam accumulation is kept to a minimum.
Bioreactor Control and Alarming
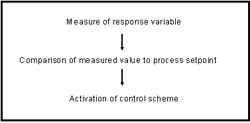
Mammalian cell culture based bioprocesses require monitoring and control of the bioreactor environment to ensure consistent bioprocess performance. The parameters requiring control include temperature, agitation, dissolved oxygen (DO) and pH. Other parameters such as cell density, nutrient concentration, desired and undesirable culture by-products are controlled indirectly via medium and feed formulation and can be greatly affected by the physical and chemical parameters. Neglecting control of these parameters could potentially impact final product quality, so online measurements can be employed to maintain the culture in an optimal state. Bioreactor control schemes entail a series of steps as outlined below.
(Click to enlarge image)
Parameter monitoring and control requires the use of an appropriate analytical device, an appropriate sampling method and a control system which can act appropriately to the information it receives. Online monitoring control systems are rapid, non-invasive and minimize potential for contaminant introduction and may be performed inside or outside of the bioreactor but must be connected directly to the bioreactor interior. Conventional parameters subject to online analyses include pH, DO concentrations, agitation, backpressure and temperature. Measurements of additional chemical parameters including cell density and viability, waste metabolites, nutrients and product concentration have historically been measured offline, although many technologies are becoming available that enable online measurement.
Online control employs probes, each of which have a sensor whose function is to gather information relevant to the biological state of the culture. This information is then converted into an electrical signal that can be amplified, recorded and analyzed so as to drive the applicable control scheme. In light of this, it is important that this information is pertinent to the current or future state of the bioprocess and be quickly generated and processed with minimal manual intervention. The sensors should be selected based on the following criteria: potential to cause contamination, robustness and reliability of sensor elements, specificity for parameter being measured and insensitivity to the harsh environment of the bioreactor. Maintaining reproducible and acceptable product quality and productivity, while minimizing downtime, are the primary business drivers behind an effective bioprocess monitoring and control strategy.
Effective bioreactor control may entail monitoring more than just the primary control parameters. For example, we once encountered a situation in which the pH stayed within the control range, but caustic was being fed to the tank even though the base controller had an output of zero such that the controller was not trying to feed caustic. Our investigation led to the discovery that the tubing from the caustic vessel to the bioreactor was not installed properly in the peristaltic pump, and caustic was leaking past the pump and into the bioreactor.
Another problem we experienced entailed hyperoxygenation of one of the seed bioreactors. This led to decreased growth, viability and increased specific lactate production. Investigation of the incident led to the discovery that oxygen was leaking into the process air line. The DO probes had been calibrated per ticket instructions but the oxygen leak led to false readings and improper DO control of the bioreactor. The only indicator, other than cell culture performance, of these false readings was the nano-Amp readings of the DO probes, which were found to be much higher than normal.
Temperature Control
Temperature is a key parameter requiring monitoring and control throughout bioprocesses to ensure an actively growing and productive mammalian cell population. In general, an accuracy of ± 0.5°C is considered adequate for cell culture, although transient excursions may exceed that range with no impact to product quality or cell culture performance. Typical bioreactor temperature measurement devices are resistance temperature devices (RTDs), which are highly accurate, reproducible and only moderately expensive. The response time of these devices is in the order of several seconds. These RTDs rely on the fact that the platinum core wire conductance varies with temperature to quantify temperature. In the RTD temperature sensor control scheme, the signal is amplified, linearized and transmitted to a controller whereupon it is compared to a setpoint. Based on this continuous comparison, the bioreactor’s temperature is regulated by adjusting the temperature of the jacket surrounding the bioreactor. If and when temperature deltas are recorded, the temperature of the jacket is adjusted appropriately through use of heat exchangers.
Dissolved Oxygen Control
Mammalian cell cultures require oxygen for the production of energy from organic carbon sources — e.g., glucose. Given oxygen’s poor solubility in water-based solutions, the control of oxygen flow is carefully regulated to ensure it does not become a rate-limiting factor in the process. In contrast, a hyperoxygenated bioreactor air supply can irreversibly and adversely impact culture performance.
Due to fluctuating cell concentrations and the associated fluctuating oxygen consumption rate, the quantity of dissolved oxygen (DO) in culture medium is in a state of dynamic equilibrium. At a constant temperature, the DO concentration in the culture media (CL) is proportional to the amount of oxygen in the vapor phase within the media (CG) in a manner that is dependent on temperature and media composition (represented by Henry’s law constant, H in the equation below).
CL = HCG
Amperometric DO probes are typically used which measure the reduction of oxygen at a cathode and the formation of silver chloride at the anode with an electrolyte solution bridging the gap between the nodes. Given the nature of amperometric DO probes, it is necessary for these probes to be allowed to polarize prior to their use. A calibration is then performed, and in the event that the probe falls outside of the acceptable calibration range, the probe membrane body and electrolyte are replaced.
pH Control
Along with temperature and dissolved oxygen control, effective pH control is vital to ensure process success given the sensitivity and potential cellular damage that may occur if pH control remains unchecked. Although cell culture media typically provides substantial buffering of pH, mammalian cell metabolism routinely decreases the culture pH due to the production of lactate and carbon dioxide, both of which are acidic in nature. Excessive hydrogen ion concentration may alter normal cell metabolism and proliferation by impairing substrate uptake and product release. In addition, it is possible that the bioactivities of some secreted monoclonal antibodies or therapeutic peptides could be pH sensitive.
Typically, the pH probes on the bioreactors are calibrated while connected to the transmitters on the bioreactor that is destined for use and prior to installation into the tanks. Typical calibrations are conducted using two buffers, with a calibration check performed in an intermediate buffer. Failed calibrations are typically due to damaged pH probes, but may also be attributed to faulty cables or transmitters.
Once calibrated, pH probes have occasionally been observed to generate incorrect readings, due to probe drifting, slowed response time or impaired sensitivity. These erroneous readings are typically attributed to sensor membrane alterations due to extreme temperature swings and fouling from media and cellular components. As a result, a policy for re-standardization of the probes may need to be developed using an orthogonal pH measurement method as the gold standard.
Effective pH control can be achieved through use of two separate PID loops, where one is the acid controller and one is the caustic controller. In a bicarbonate-buffered system, the acid controller controls the carbon dioxide flow and is configured such that the carbon dioxide flow ramps up very quickly when the process value is above set point and instantly turns off when the acid controller set point is reached. The liquid caustic controller utilizes a pulse width modulator (PWM) to control the amount of time the caustic peristaltic pump is on or off. The set point on the peristaltic pump is set manually per manufacturing ticket instructions and the controller only turns the pump on and off. The frequency of measurement and pulse addition and duration can be altered to effect varying levels of control by tuning the control loop. Due to the high pH of the caustic feed, it should be fed into the bioreactor through a sub-surface port to facilitate quick dispersion into the culture.
Dissolved Carbon Dioxide Control
Dissolved and evolved (i.e., headspace) carbon dioxide levels can be indicative of cellular metabolism and are thus routinely monitored as indicators of culture performance. In general, the mammalian cell cultures display sensitivities to extremes of dissolved carbon dioxide (pCO2) be they low or high. High pCO2 levels have been reported in the literature as an inhibitor of growth and metabolism and can impact product quality characteristics such as glycosylation of the protein product.
Several parameters can affect the pCO2 levels, including pH set point, temperature, sodium bicarbonate concentration, cellular metabolism, caustic addition to the medium and gas flows. Each of these parameters must be considered carefully to enable successful pCO2 control. pH, temperature and bicarbonate concentrations are typically not adjusted during a process to control pCO2, but rather gas flows and caustic addition are controlled to maintain the pCO2 within the desired target range. The gas flows can be chosen to strip out the desired amount of dissolved carbon dioxide as experience has shown the carbon dioxide levels are influenced more by total gas flow through the bioreactor rather than kLa.
If the culture pH has drifted to the acidic side of the dead band, increasing the airflow strips out carbon dioxide potentially leading to an overall reduction of caustic addition. The reduced amount of caustic can lead to a lower pCO2 at the end of the culture when lactate levels typically decrease. However, if the pH is on the basic side of the dead band such that the CO2 is being fed, increasing the airflow will only lead to increased CO2 flow and will not affect the pCO2.
Backpressure Control
The stainless steel bioreactors are maintained under positive pressure to create an environment that is more conducive to axenic operation. A backpressure setpoint is generated by maintaining a constant overlay process air flow into the headspace of the bioreactor. The backpressure can then be controlled via a PID control loop that operates a flow control valve on the vent line. To avoid safety concerns associated with over-pressurization, rupture discs may be incorporated into all pressurized stainless steel vessels to act as pressure relief devices. In addition to the bioreactor headspace, positive pressure should be maintained on all transfer lines within the sterile boundary and any associated auxiliary stainless steel vessels used for additions to the bioreactors.
Backpressure can also be used as the driving force to govern bioreactor-to-bioreactor transfers and bioreactor-to-primary recovery transfers. Care should be given to ensure the transfer is fast enough to not allow cells to settle during the transfer, but not so fast as to subject the cells to excessive shear. The transfer time can be dictated by the pressure drop and pipe dimensions.
Alarm Strategy
The alarm strategy should be configured to alert the operators that the process is deviating from its acceptable range, but should also provide early warnings such that the operator can respond in time to prevent loss of the batch. To this end, multiple levels of alarming may be implemented. The first level of alarms can be set to include the normal variability present within a control loop such that if the alarm is activated, operations can assume that an unexpected excursion has occurred but will have time to take pre-emptive action before the process is negatively impacted. The final level of alarms should be set to match the acceptable ranges listed in the process flow chart specific to a process. While determining the alarm strategy, care should be taken to apply alarms only to the appropriate parameters. If excessive alarming occurs, operators may begin to not respond effectively to alarms to the extent that important alarms may be missed.
Use of Offline Data
Additional information regarding culture health and performance may be obtained offline using analysis of aseptic samples of the culture. Typical offline testing will yield information regarding cell numbers and cell viability using an automated cell counter system. In addition, a blood gas analyzer can be used to determine the levels of relevant parameters including lactate, glucose, pCO2 and pH. The offline samples may be used primarily for informational purposes and not linked into automated response systems to drive bioreactor control changes. However, offline measurements may be used by technical services to monitor the process and instruct operators to, for example, restandardize the pH probes should a drift from setpoint be observed.
Bioreactor Feeding Strategy
The basal medium may be added to the bioreactor from a disposable bag via peristaltic pump, or for larger volumes from stainless steel media make-up tanks via pressure transfer. For the tank transfers, the media is typically sterilized in-line via two hydrophilic filters, a pre-filter followed by a sterilizing grade filter. The filters are steam sterilized in line simultaneously with the transfer path and are cooled prior to media transfer.
Nutrient feeds are typically prepared in disposable bags. The nutrient feed typically consists of multiple stock components that need to be well mixed and may require pH adjustment. Upon one nutrient feed into a bioreactor, a high pH excursion was observed in the bioreactor which was later attributed to poor mixing of the nutrient feed components. The nutrient feed bag was subsequently re-designed to allow for better mixing within the bag to avoid the pH change in the bioreactor.
Nutrient feeds are delivered to the bioreactors at pre-determined times during the cell culture process. These feeds are manually added to the bioreactors, with very little automation associated with them. In fact, the automation is configured such that the near-to block valve on the nutrient feed line is always open during culture phase to maintain positive pressure on the line. Therefore, the peristaltic pump head is the only block on the line, such that the operator can initiate the feed simply by turning on the pump. The nutrient feeds may be slightly acidic, so a typical concern associated with addition of the feed is a change in pH in the bioreactor. To account for this, the addition flow rate of the feed is dictated, as well as instructions to the operators to stop the feed if the pH exceeds the acceptable range.
About the Authors
Cillian McCabe has a first-class honours degree in Biotechnology from National University of Ireland (NUI), Galway, and a PhD in “Gene Therapy Approaches to the Treatment of Type 1 Diabetes Melitus” from the School of Medicine at NUI. He joined Eli Lilly & Co. in 2007 and has supported Bioprocess Development and Manufacturing Operations both in the U.S. and Ireland as part of Lilly’s Manufacturing Science & Technology functional group.
Brian Stamper has a B.S. in Biochemistry from Indiana University (Bloomington, Ind.) and an M.S. in Biological Engineering from Purdue University (West Lafayette, Ind.). He joined Eli Lilly in 2001 as a development scientist in Bioprocess Development and transitioned to Bioprocess Operations in the Clinical Trial Material Supply pilot plant in 2005 as a manufacturing associate.