From Drinks to Drugs: A Proposal for Advancing Aseptic Processing and Eliminating Contamination Risk
For most of the last three decades, there has been a tacit understanding that one simply had to accept some level of contamination in any aseptic processing environment. The reasons for this begrudging acceptance are easily understood.
First, for most of those three decades gowned human operators have been an absolutely essential requirement in aseptic processing. Second, the very presence of personnel necessitates a human-scale clean room—one that allows people to conduct the work of moving components and supplies to points-of-use, set up equipment, and make the necessary (and risk-intensive) interventions that have been necessary to the conduct of aseptic processing. Third, although less significant than people working in close proximity to the process and product, human-scale clean rooms need points of entry and exit for personnel and also rely on manual disinfection of materials that may enter the aseptic operations area through airlocks of various design.
Although in the modern human-scale clean room these entry points are typically fastidiously designed and carefully controlled, they remain a secondary source of contamination and, while rather minor in comparison to human-borne contamination, can still cause contamination problems that are difficult to detect and can put product at risk.
Of course, as the years have progressed regulations for the aseptic production of pharmaceutical products labeled sterile have continued to tighten. A perusal of either the current FDA guidance regarding aseptic processing or Annex 1 of the EU GMP regulations clearly confirms that regulatory expectations are much higher than they were even a decade ago. The authors believe that this ongoing escalation in regulatory requirement for aseptic processing originates, at least in part, from the perceived need of the authorities to encourage firms to make the closest approach possible to an absolute proof of sterility. Certainly, while it is clear from a scientific perspective that an absolute proof of sterility will always elude us, it nevertheless seems unlikely that the regulatory trajectory of the last three decades will change.
That being said, the wise course in strategic planning for aseptic processing operations must include consideration of not only current but future regulatory requirements as well as product liability. Thus, it is prudent to consider not only contamination risk to the product, but also producer’s risk in the event that contamination issues that are difficult to detect and resolve should arise. It seems safe to suggest that a production system that has an exceedingly low likelihood of contamination risk in terms of media fill, sterility test or environmental monitoring would provide the greatest abatement of producer’s risk in terms of both compliance and increasingly legal liability.
Production Ease and Efficiency vs. Contamination Risk
In our experience, the strategic decision regarding the production environment for aseptic processing these days most commonly hinges on a trade-off between state-of-the-art contamination control and production ease and efficiency. Since at least the early 1990’s, it has been recognized that isolator technology affords contamination control advantages over conventional human-scale clean rooms. However, although there have been numerous examples of successful implementation of isolator technology, the perception among firms conservative in adopting this technology is that it was difficult to design, hard to validate, and could result in severe compromises in terms of operating efficiency.
Among the most prevalent concerns relating to efficiency have been difficulty in making adjustments to correct operational faults, ergonomic concerns, problems in supply and component movement into and out of the isolator. Additionally, there have been fears regarding cumbersome (and lengthy) changeover from one container size or configuration to another.
These issues ultimately led to some isolator systems actually being decommissioned, and restricted access barrier systems (RABS) being considered a means of achieving most of the contamination control isolators offer while retaining some, or perhaps even most, of the human-machine interface advantages of a clean room. RABS, though (unless it is the closed variety, which is operationally very much the same as an isolator), does not diminish contamination or producer’s risk as effectively as isolators. So-called open-door RABS certainly was the subject of considerable enthusiasm within industry a few short years ago. However, at present it is possible (perhaps likely) that this approach is best suited to the improvement of existing aseptic processes.
So, while nearly all new installed aseptic pharmaceutical production systems embody some form of what can be called separative technologies such as isolator technology, RABS or even some combination thereof; the selection of environmental technology continues to be a major, perhaps even the major, point of discussion and debate during the conceptual design phase of aseptic production projects.
A Relevant Case Study from Another Industry
A widely held view in the pharmaceutical industry is that the technologies employed in this industry sit at the pinnacle of aseptic processing. Certainly, the authors do not mean to suggest that the knowledge base and professional capability in the pharmaceutical industry is anything other than outstanding. However, based upon our experience, we believe that the pharmaceutical industry can learn from the directions taken by the aseptic beverage industry over the last 15 or so years.
In the beverage industry, there is no debate at the present time regarding aseptic manufacturing environments. All such environments are separative enclosures similar if not identical in all respects to what are known in pharmaceutical aseptic processing as isolators. Of course, since the regulations are different, some important differences exist, perhaps the most noteworthy of which is the use of turbulent air flow systems instead of unidirectional (“laminar”) airflow. However, air entering the enclosure is always filtered through HEPA or in some cases ULPA filters. As one would expect given this level of air filtration, there is no difference in contamination risk arising from the use of turbulent air flow rather than unidirectional flow.
Decontamination of the Enclosure or Isolator
Decontamination of the isolator in aseptic beverage filling is similar in all respects to the sporicidal treatments used in conjunction with pharmaceutical isolators. The most common agents used are hydrogen peroxide (H2O2) or a combination of H2O2 and peracetic acid. The equipment built, installed and validated by Shibuya Kogyo Co. relies upon H2O2 either in liquid form or vapor phase. As is the case with pharmaceutical isolators, decontamination efficacy is confirmed by biological indicator challenges conducted using bacterial spores, and the expectation is complete kill of the challenge spores.
The approaches are somewhat different, but in our experience the outcome is effectively the same: Both the beverage and pharmaceutical aseptic processing isolator, when decontaminated, do not have any apparent microbiological contamination within them.
Why Aseptic Beverage Production?
The reader may be wondering why a beverage would be aseptically produced. First, many beverages are at substantial risk from microbial contamination. Drinks that are low acid or contain milk are, because of their nutrient content, subject to microbial contamination and hence spoilage. The use of aseptic processing can improve flavor, reduce the need for continuous cold storage in shipment and increase the shelf-life of contamination-sensitive products. Aseptically filled products, since they typically do not contain anything in their formulation that is bacteriostatic or bacteriocidal, are very spoilage evident. As a result, it is quite possible to get a realistic picture regarding the likelihood of contamination appearing in the field. In the United States, the FDA regulates aseptic beverage production, both in terms of approval of manufacturing processes and ongoing plant inspection.
Process Description and Background
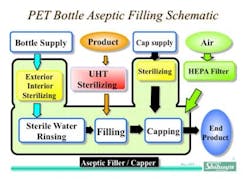
In the 1990’s, Shibuya Kogyo developed a new range of equipment for the aseptic production of beverages in light, clear PET plastic bottles. As of the writing of this article, there have been more than 50 such processing lines based upon this concept installed in Asia as well as the United States, where FDA approval has been achieved. While the fundamental layout and processing approach have adhered to common technological principles, there have been evolutionary improvements and customization to satisfy the needs of specific customers. A generalized process flow for aseptically filled beverages may be seen in Figure 1 below.
One of the key considerations from the outset was that these aseptic beverage processes would have to be extremely high in output and very reliable in operation. The throughput speeds desired by customers have typically ranged from around 600 bottles/minute to as high as 1200 bottles/minute. Given that the typical fill volume range for these products is approximately 300 mL to a liter or more, the throughput rates require large multi-head filling systems of rotary design. Additionally, a gravimetric net weight filling system was desired for optimal process control. The size of these systems and the speed made it necessary that the systems be sufficiently stable in operation that routine interventions were eliminated. In fact, given the rotary speed and mass, interventions could only be safely done if the equipment were fully stopped. Therefore, a key principle in equipment design had to be extremely stable and reliable operation without jams.
Also, prospective users desired extremely high rates of “up time”. Since 24/7 operations were desired, mechanical reliability as well as process reliability were absolute requirements. As a result, the following requirement specifications were established:
- The lightweight PET bottles would be neck-handled to ensure stability at high speed through positive bottle control. Therefore, simple, reliable and precise high quality grippers were designed to handle the speed and post-fill weight of the bottles. These grippers can be easily changed out for service during infrequent process stoppages. The neck handling strategy also makes it easy to convey bottles within the system without jams or misfeeds and to “hand off” bottles from one rotary station to another.
- The grippers within the filling section are attached to lever arms acting on load cells so that each bottle could be filled to a precise weight. Filling nozzles and rates can be customized to minimize foaming. Fill data is output to a PLC/computer as a permanent record.
- Bottles and caps are sterilized in-line and enter the fill enclosure ready for use; thus only sterile materials enter the isolator and no human interventions are required to periodically introduce parts into the filling system. All component feeding is fully automatic. Bottles may be sterilized with H2O2, although more recently electron beam sterilization of bottles has been employed with superb results. Some systems have incorporated blow molding of the bottles, with sterilization occurring post molding. Caps are also sterilized by H2O2. Bottle and cap sterilization is accomplished at a fast enough rate to keep up with filling speed.
- Capping of filled bottles is done using a servo-capping system that tightens the caps to a predetermined torque value.
- Any product that falls outside the established acceptance criteria for weight, cap torque or appearance is automatically rejected with no human intervention required.
- The isolators can be considered during product use to be “gloveless”, and gloves when installed can not be used for intervention during production operations.
- The entire fluid or wetted path through which the product passes on its way to the fill nozzles is both cleaned and sterilized in place (C/SIP). These C/SIP steps are fully automatic and thermocouples provide control and process monitoring during moist heat sterilization.
- The isolator enclosure can also be “CIP’ed” using a cleaning process optimized for the product(s) being aseptically produced. The isolator enclosures are designed for optimal CIP efficacy. Spray ball location is carefully considered, as is effective drainage of cleaning solutions. The processing equipment is designed so that sensors and sensitive electronics are well protected from the CIP and decontamination processes for maximum reliability and longevity.
Figure 2 provides a view through an isolator enclosure window into the filling section. Several bottles can be seen in various stages of filling. The rotary filler uses C/SIP capable gravimetric weight-controlled fillers, and may have as many as 120 filling heads.
Figure 2. A look inside: aseptic bottle filling via rotary filler, which may have as many as 120 filling heads.
A key operational objective in designing this advanced aseptic processing system is that it would require so little direct human interface in operation that it could operate essentially “lights out”. As such, the human interface with the equipment is primarily at a PLC/computer video screen. Thus, a plant equipped with multiple fill lines of this design might require only a small number of technicians and operators to control and monitor these advanced aseptic processing lines.
Direct Applicability to Aseptic Pharmaceutical Manufacturing
We believe that nearly all of the technology embodied in this highly automated, very high-speed aseptic beverage system can be directly applied to pharmaceutical manufacturing. Neck handling, for example, is directly applicable to vial and plastic bottle manufacturing. Fully automated rubber stopper sterilization and feeding have been accomplished using a combination of automated sterilization and H2O2-resistant robotics systems. Vapor phase hydrogen peroxide parts feeders have also been developed and can be interfaced with decontamination pass boxes to allow fully automatic, contamination-risk-free introduction of components such as caps, dropper tips and bottles that are radiation sterilized in bags. In the near future, sterilization of plastic components in line seems within reach.
Conclusion
The proof of any production process is in the quality of the end product. Variations on the basic aseptic beverage filling process described in this article have produced well over seven billion containers without a single report of contamination. As previously mentioned, the products filled are not preserved and most are quite supportive of microbial growth. We believe this result, along with the very high yields obtained, clearly demonstrates the capability of highly automated and very advanced aseptic manufacturing systems to achieve very high levels of contamination risk abatement without compromising operational efficiency and reliability.
These systems have the potential to not only revolutionize the manner in which aseptic processing is done, but also to completely change industry and regulatory thinking regarding process control, process validation, and environmental monitoring. In fact, environmental monitoring, where required, can also be fully automated using vapor phase hydrogen peroxide-resistant robotics. Fully advanced and optimized aseptic processing that comes very close to the theoretical limit regarding elimination of contamination risk is not only available in 2009, it is very efficiently at work everyday supplying the optimum in microbiologically pure beverages and pharmaceutical products.
About the Authors
James E. Akers, Ph.D., is President of Akers Kennedy & Associates, Inc. (Kansas City, Mo.) Dr. Akers has over 21 years experience in the pharmaceutical industry; he has worked at various director-level positions within the industry and for the last decade as a consultant. He served as president of the PDA from 1991 to 1993, and as a member of the PDA Board of Directors from 1986-1999. Currently, he is chairman of the USP Committee of Experts for Microbiology and Sterility Assurance; he is a current member of several PDA task forces working on revisions of technical reports. He is also an ISPE member and lectures frequently at ISPE symposia. Dr. Akers can be reached by e-mail at [email protected]/
Yoshi Izumi graduated from Kanazawa University in Kanazawa, Ishikawa Prefecture Japan in 1982 with a degree in Mechanical Engineering. Mr. Izumi joined Shibuya Kogyo, Co. LTD in 1983 and worked as a Mechanical Engineer through 1984. He was then transferred to the United States where he served as a Sales Engineer for Shibuya stationed both in New Jersey and California from 1985-1995. In 1996, Mr. Izumi returned to Japan where he became General Manager in the International Marketing Department at Shibuya. In 2006 Mr. Izumi moved again to the United States as Executive Vice-President of Shibuya Hoppmann Corporation in Elkwood, Virgina. Mr. Izumi has participated in several projects involving Shibuya’s advanced high speed aseptic beverage systems both in Japan and the United States, and has also been involved in projects relating to pharmaceutical aseptic processing in both Japan and the United States as well.
References
- Task Force on Sterile Drug Products Produced by Aseptic Processing (with support from the Japanese Ministry of Health, Labor, and Welfare). Guidance for Industry—Sterile Drug Products Produced by Aseptic Processing, 2005.
- Oshima, Y., Yoshida, S. and J.E. Akers. PasepT: A New Concept in Aseptic Processing, in proceedings of the 36th R3 Nordic Symposium and 5th European Parenteral Conference, Linkoping, Sweden, 2005.
- Akers, J.E., Kokubo, M., and Y. Oshima. The Next Generation of Aseptic Processing Equipment. Aseptic Processing Supplement to Pharmaceutical Technology 2006: 32-36.
- U.S. Food and Drug Administration. Guidance for Industry. Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practices, 2004.
- Akers, J.E. An Overview of Advanced Aseptic Processing—and a Few Thoughts on Implementation. Proceedings of the PDA Conference on Risk Management and Aseptic Processing, May 2008.
- Akers, J.E., Tanimoto K., and M. Kawata. Aseptic Processing the Japanese Way, Pharmaceutical Manufacturing, June 2006: 23-27.