Lechleiter
At press time, it was announced that John Lechleiter, currently president and COO of Eli Lilly and Co., will take over as chairman and CEO of Lilly when current CEO Sidney Taurel resigns on Mar. 31. Judging from Lechleiter's Nov. 29 presentation at Harvard Medical School’s Personalized Medicine Conference, Indianapolis-based Lilly will be piloted by an able and confident captain who doesn’t mince words.
Praising conference organizers for bringing together such an illustrious group of people from many segments of the healthcare industry, Lechleiter added, “I’m pleased you saw fit to include someone like myself from a large pharmaceutical company. Personalized medicine will change healthcare almost across the board, and nowhere, I would argue, are the crosscurrents of change going to be more powerful, or the stakes higher, than in the development, manufacture and sale of prescription medicines.
“In my industry, we would be powerless to resist personalized medicine, not to say foolish," he continued. "For that reason, we at Lilly remain somewhat mystified by the skepticism that persists around the questions, 'Are the drug companies really on board with personalized medicine?' and 'Will their business model accommodate products more finely targeted to patient groups within a disease category?' "
He added, "Now I can only speak for Eli Lilly and Co., but my answers to those questions are 'Yes, we’re not only on board, we’re also trying, in recent years, to serve as a kind of conductor to help put others on board' and 'Yes, our business model will accommodate personalized medicine — in fact, it may depend on it.' "
Contrary to popular perception, the pharmaceutical industry has rarely pursued ‘one size fits all’ in its development or promotional efforts, Lechleiter argued. "What we call segmentation of disease categories or patient groups has long been the norm," he explained. What’s fundamentally different today, he said, is the availability of tools, technologies and new knowledge to inform such segmentation, and to render it more relevant and more meaningful.
Lechleiter pointed to biomarkers as a good example of such tools. Although these "telltale indicators of biological activity to help guide diagnosis and treatment decisions are nothing new," he observed, now they are more pervasive and more sophisticated than ever before, and are coming into play at much earlier stages of drug discovery and development.
"Today at Lilly, fully 90% of the molecules reaching the clinical stage of development have a biomarker strategy associated with them — a dramatic increase over the past few years," Lechleiter observed. "That’s already generated some important benefits for us in our R&D processes, allowing us to weed out unpromising molecules earlier in the game, to compress development timelines, to run smaller and more focused clinical trials, and to adapt them midstream, in some cases, based on what we’re seeing and to start exploring secondary indications more quickly."
He said Lilly executives hope that over time, widespread application of biomarkers will result in shorter cycle times and lower costs in drug development. "I personally believe this is critical," he stressed.
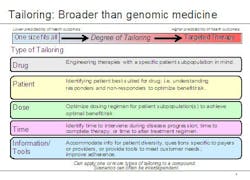
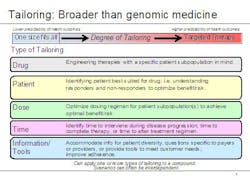
Lechleiter noted that the growing use of biomarkers is just one aspect of personalized medicine. To capture the broader significance of where the company sees healthcare going, he said, Lilly executives have coined the term 'tailored therapeutics.' "The notion of tailoring, we believe, goes beyond typical understandings of personalized medicine in two important ways," he explained. "First, there are different types of personalized healthcare, and second, there can be wide variation within the degree of personalization." He used the graphic below to illustrate his point.
Examples of tailored therapeutics at Lilly include:
- A designer molecule for poor responders to another therapy (AME molecule for people who don’t respond to Rituxan)
- Earlier, better information – during drug development – on patient groups with higher risk factors (e.g. Xigris)
- A companion diagnostic developed six years after a drug’s first launch
- Data from clinical practice used to identify patient groups who will benefit most from a particular therapy, for example, Strattera. ADHD is often treated with stimulants such as Ritalin, but for some patients, stimulants don’t achieve the desired effect. Strattera is the only non-stimulant drug that is FDA-approved for the treatment of ADHD.
Why is personalized medicine needed?
Noting that some people, both within and outside the drug industry, will undoubtedly question the need for personalized medicine, Lechleiter cited several reasons:
- About half of all patients fail to respond to a drug they’re prescribed (see graphic below)
- Health care products and services are viewed by consumers as relatively low in value
- The risk-benefit ratio is under scrutiny for many [and all new-to-market] drugs.