A Development Roadmap for Combination Products
Over the last decade, we have seen a growing number of innovative products combining the benefits of technology with drug and or biologic therapies. Combination products can range from the relatively simple, such as pre-filled syringes or injector pens, to highly complex systems (e.g., drug-eluting cardiac stents).
In 2003, Johnson & Johnson launched the first drug-eluting stent, thereby challenging the industry and FDA to establish a model for novel products balancing business and public-safety requirements. Since then, we have seen an influx of new and innovative therapies pushing the boundaries of the therapeutic and regulatory landscapes. Combination products seek to leverage the best of the device performance world in an effort to maximize therapeutic performance.
With this quest comes a new challenge. The development philosophies for devices and drugs are not the same, and marrying the two technologies carries its own unique set of challenges. In a marketplace in which Time-to-Market (TTM) defines what we do on a daily basis, the business goal often shifts to meeting deadlines as opposed to assuring the quality of the task. This represents a problem for all regulated systems but the impact of cutting corners in a combination product can be profound, both to the customer and the organization.
Execution must include a measurement of capability as part of the completion assessment. How do we meet the conflicting demands of TTM, cost and completeness? One solution is to borrow from proven operational excellence methodologies that have demonstrated their ability to optimize new product development effectively within both the drug and device industry.
This article discusses the technical considerations in developing a combination product and proposes a technology roadmap, leveraging the principles of Six Sigma and Lean Manufacturing for integrating the technical and quality elements in the development of combination products in the U.S.
Figure 1. Software, Device and Drug Lifecycles
Collision of Lifecycles
From a pure regulatory perspective, the challenge in developing a combination product is multifaceted. Depending on the product, there is likely to be combined oversight from both CDRH and CDER or CBER. This level of complexity raises the stakes during the product development lifecycle, because it potentially brings together three similar but not identical development philosophies. The basic elements of each lifecycle are shown in Figure 1 (above).
Embedded within each lifecycle are the fundamental quality elements necessary to ensure that the product performs predictably. The basic expectations are defined in 21CFR 820 and 21CFR 210/211. Add to this the requirements of ICH Q2, Q7, Q8, Q9, Q10 and ISO 2000/CE marking, and the potential for misstep is tremendous.
Successful programs integrate downstream considerations as early in the product-development lifecycle as is possible. Integrating business requirements in the concept phase can save an organization years of development time and millions of dollars, simply by not chasing a product design that is either not viable or not a frank commercial success.
Lean Six Sigma and the Development Roadmap
One key difference in developing a combination product is the necessary mindset shift from two individual components (device and drug) to a focus on combined performance. Applying a Six Sigma roadmap provides a structured framework to identify the key process input variables that control overall product performance, not just device or drug performance. The Six Sigma roadmap uses a fivephase project management process to drive improvement: Define, Measure, Analyze, Improve and Control (DMAIC). Step by step, each phase in the DMAIC process guides the members of the development team through the project in a way that provides the right data and best process understanding. Managing the project in this way allows the business to make the best possible decisions with the available data and resources. The concept behind the process is as follows:
- Define: Clearly define the problem and relate it to the customer’s needs.
- Measure: Measure what is key to the customer and know that the measure is accurate.
- Analyze: Search for the root causes and identify the most likely causes.
- Improve: Determine the root causes and establish methods to control them.
- Control: Monitor and make sure the problem does not come back.
A set of deliverables must be completed for each DMAIC phase to ensure all project requirements are met. As we evaluate applying Six Sigma principles to the development of a combination product, there are several models to consider. The classic Six Sigma DMAIC model provides a good framework for objective scientific inquiry, typically used to improve existing processes (and products). However, a Design for Lean Six Sigma (DFLSS) approach, with its focus on the development of new products and processes, would be more appropriate for this application.
DFLSS models provide a structured, phased approach to the design of a product, process or service, with Six Sigma Quality (target of 3.4 defects per million opportunities) and efficiency as key design criteria. Also, it’s easy to include risk management in the model, as Failure Modes and Effects Analysis (FMEA) is a standard DFLSS tool.
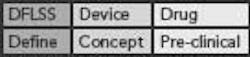
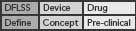
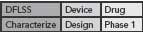
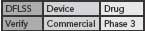
This DFLSS tollgate approach to product development has the additional advantage of providing a set of success criteria for the completion of key milestones within each phase of the process (so that the “gate” can be closed). Comparing progress against such criteria provides objective evidence of incremental team success (that can be celebrated and communicated to the rest of the organization) and ensures that the somewhat asynchronous development philosophies of devices and drugs are being considered appropriately. Possible DFLSS frameworks include the DMADV, IDOV and DCOV models. These are shown in Figure 2 (p. 36).
Any of these models will serve to meet the needs of a development program, but the DCOV DFLSS model, with its focus on process characterization and optimization, is a particularly good fit given the drug development component of the product development lifecycle. The following sections map the DCOV model to the phases of the drug and device development model.
Toll Gate 1
At the outset of the development program, it’s imperative to develop a clear product specification. This specification should define product performance, user-interface requirements and product hazards for both the device and drug. Typical criteria to include, in addition to performance specification, may be drug shelf life, overall system cost targets (initially and at volume), product safety requirements and marketing attributes. Establish reliability goals along with a technology risk assessment prior to the actual device design effort.
Figure 2. Possible Design for Lean Six Sigma frameworks
In the early phases of product development, the software and device programs will concentrate on proofof- concept activities. The device component focuses on functionality as it pertains to performance with the drug. Within the drug development lifecycle, the drug and device system may be a tabletop-sized unit capable of delivering a therapeutic dose.
Analytical methods are in their early development stages and no equipment or methods have been qualified. If the device requires application software or embedded software, the presence of a structured software development and quality assurance plan will be required. The drug activities will revolve around basic formulation activities, early stability and Pk / PD studies. Opportunities exist at this stage to characterize the drug / device integration with the intent of identifying key process input variables that may impact product performance.
Toll Gate 2
In the next phase, the device and drug development efforts focus on meeting the product specifications established in the Define phase. Translating the customer requirements into Critical to Quality (CTQ) attributes, and ultimately Critical Customer Requirements (CCR), that must be measured and controlled within the process, should be established. Within the product performance assessment, an error allocation and tolerance stack-up analysis also must be performed. This requires a minimum acceptable process capability metric in order to ascertain what the actual equipment capability must be. A common mistake is to delay this activity until commercial development. The risks of pursuing a design that will not meet the requirements of the product specification escalate as they progress along the development lifecycle.
This phase entails intensive modeling and risk assessment activities. In the Design Phase for the device, Finite Element Analysis, reliability blocks, FMEA, thermal analysis and DOEs are routinely applied. The drug will begin its QbD characterization activities as the process, specifications and quality oversight increase in order to satisfy 21CFR 210 cGMP requirements. Software quality assurance elements will be required for all application and embedded code developed. These software development models can follow any number of schemes including the Capability Maturity Model or Waterfall method.
Toll Gate 3
In the Optimize phase, the quality infrastructure for both the device anddrug are increasing. At this juncture, a plan must be developed that allows the systems to be released that can serve as a foundation for commercial production. This is more difficult than it sounds. Within the device world, releasing devices against gold standards (i.e., systems that display very little variation) is not unusual. There is no equivalent to this in the drug world. At the core of the dilemma is the regulatory strategy. If the filing is an NDA for both the drug and the device, then the quality argument must be able to defend releasing a lot of devices and drugs together as a system.
At this point, the device is going through intensive development and testing. Typical testing includes: Highly Accelerated Life Testing (HALT), fracture and fatigue, design verification testing, environmental testing, package design and verification testing. In addition, the reliability plan should be finalized in order to generate data to support the reliability requirements for the system. These requirements will vary with the criticality of the therapy. For example, if the system is delivering insulin to a Type 1 diabetic, the FDA reliability requirement will be very high because the consequence of offspec performance or non-performance could be life-threatening.
The drug also will begin scale-up to intermediate-stage manufacturing, usually employing semi-automated manufacturing equipment. Validation creeps into the picture at this point, as the quality system requirements (QSR) escalate prior to Phase 2 clinical trials. Facility, equipment, personnel and methods all will need to be qualified prior to Phase 2.
Toll Gate 4
This is the final step in the development process. The quality system now reflects a commercial process and must be fully compliant with CFR 820 and CFR 210. All key process parameters associated with system performance have been identified, and key output parameters have been defined and measured. Demonstrating consistency from each phase in terms of the key output parameters is central to making the claim that the clinical performance measurements are valid. The device reliability program shifts to execution measurement systems utilizing Highly Accelerated Stress Screening (HASS) tests, root-cause analysis tools and supplier qualification programs.
The drug will mirror the same requirements for demonstrating consistency between key output variables for each phase and final inprocess and release-testing methods. Statistically justified sampling plans are required to demonstrate that the process is in control prior to the final process validation. In this phase, supplier qualification programs focusing on process capability and measurement system sensitivity are finalized and tracked in order to ensure that the product can consistently meet the product’s release criteria.
Conclusion
The DFLSS DCOV (design/characterize/ optimize/verify) model offers a framework for objective assessment and measurement of the key development activities for a combination product. The current regulatory shift in mindset to a more scientifically driven, risk-based framework has transformed the expectations for product development. Following a DFLSS model will help ensure that organizations address both the drug and device development criteria essential to satisfying both the QSR and cGMP requirements with a focus on business performance and time-tomarket sensitivities.
References
- Kourti, Theodora, Ph.D., “Process Analytical Technology and Multivariate Statistical Process Control: Wellness Index of Product and Process – Part 1,” PAT Journal, Volume 1, Issue 1, September/October 2004, pp. 13-19.
- Kourti, Theodora, Ph.D., “Process Analytical Technology and Multivariate Statistical Process Control: Wellness Index of Product and Process – Part 2,” PAT Journal, Volume 2, Issue 1, January/February 2005, pp. 24-29.
- Kourti, Theodora, Ph.D., “Process Analytical Technology and Multivariate Statistical Process Control: Wellness Index of Product and Process – Part 3,” PAT Journal, Volume 3, Issue 3, May/June 2006, pp. 18-24.
- Harry, Mikel and Schroeder, Richard, Six Sigma: The Breakthrough Management Strategy Revolutionizing the World’s Top Corporation, Doubleday, 2000.
- Pyzdek, Thomas, The Six Sigma Handbook: A Complete Guide for Green Belts, Black Belts and Managers at All Levels, Revised and Expanded, McGraw-Hill, 2003.
- Brue, Greg, Design for Six Sigma, McGraw-Hill, 2003.
About the Author
Bikash Chatterjee is president of Pharmatech Associates, Inc. He has been involved in the biopharma, pharmaceutical, medical device and diagnostics industry for over 20 years. Most recently he served as vice president of pharmaceutical operations for Aradigm Corp., where he was responsible for establishing their process development, engineering, validation, facilities and manufacturing capabilities. Chatterjee is a certified ISO 9000 Lead Assessor, a Six Sigma Master Black Belt and has over 15 years experience in the implementation of Lean Manufacturing programs in the life sciences industry. He holds a B.A. in Biochemistry and a B.S. in Chemical Engineering from the University.