Using NIR Spectroscopy for Raw Materials Characterization
Introduction
The utilization of near-infrared (NIR) spectroscopy for the quality control (QC) of pharmaceutical tablets has become increasingly widespread over the past decade. This is due in part to stricter regulatory control of QC in the pharmaceutical manufacturing environment, but also the increasing realization that it may no longer be practical to use a wide range of testing methods within one manufacturing plant.
Chapter 5.30 of the Good Manufacturing Practice (GMP) guidelines specifies that a pharmaceutical company must provide suitable procedures or measures to guarantee the identification of the material contained in each recipient. Traditionally, companies used a number of different analysis methods for the testing of individual raw materials as suggested in early versions of Pharmacopeia methods. However, a number of new regulatory guidelines now advocate that near-infrared (NIR) spectroscopy is the best universal method for raw material testing.
By adopting NIR spectroscopy for QC purposes, pharmaceutical companies can now make significant time and cost savings in raw materials characterization. This article will explore how and provide a review of Abiogen Pharma S.p.A and its use of NIR spectroscopy for raw material analysis.
Characterization of Raw Materials
Raw material analysis is an essential process in any pharmaceutical manufacturing laboratory. According to Annex 8, EU GMP:
In compliance with GMP guidelines the identification of the starting raw materials must be executed on each API and excipient. As most pharmaceutical manufacturing laboratories often receive a huge amount of raw materials, this can be a difficult job in terms of time and cost. Scientists are often required to spend time away from the laboratory in order to carry out raw material analysis, resulting in decreased productivity, and the administrative burdens of carrying out several tests on each batch of raw material can significantly increase laboratory workloads.
Regulatory Guidance
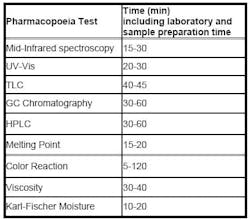
Pharmaceutical companies have the option to comply with traditional Pharmacopoeia methods for raw material identification. Prior to the European Pharmacopoeia 5th edition (2002), there was no universal method recommended by pharmaceutical guidelines relating to the analysis of raw materials. Different methods were specified for each raw material to be identified, meaning that a number of different analyses were conducted using different identification methods. Analysis times differ significantly between methods and can cause the characterization of the raw materials to be extremely time-consuming (see Table 1).
Table 1: Individual methods of raw material analysis, with time typically taken for each analysis.
Individual methods of raw material analysis with time typically taken for each analysis. Using individual methods for each raw material analysis can also incur significant costs, including the cost of different instruments, reagents and other chemicals resulting in decreased productivity of laboratory instruments and personnel. This is further amplified by the overall increase in the amount of raw materials containers received by many pharmaceutical manufacturing laboratories. Pharmaceutical companies often have a huge range of raw materials to identify, and therefore it is difficult to standardize the method used for analysis, as the type of raw materials entering the laboratory is often subject to change.
Traditionally, raw material identification was performed on a statistical basis, where only a certain percentage of each batch of raw materials would be analyzed. Although this method was advised by certain guidelines, many pharmaceutical companies have begun to identify all of the containers of the actives but not of the excipients, in order to carry out a more in-depth analysis. However, there is now a requirement to identify every single container of each batch of raw materials, and to analyze containers of both the actives and the excipients. The only exceptions to this requirement are sucrose, talc and sodium chloride, all of which are produced in mono production; therefore it would not be possible for a manufacturer to supply an incorrect raw material.
Additionally, when using traditional Pharmacopoeia methods for raw material analysis, often more than one test is mandated, for example when analyzing lactose it may be necessary to use three different methods: IR spectroscopy testing, thin layer chromatography tests and analysis using melting point.
It is evident that where there are a large number of raw materials that need to be analyzed, the use of multiple analysis methods isnt practical. There are three possible solutions to this challenge, for example mandating that suppliers provide larger size containers, validating suppliers, or finding an alternative method of analysis.
A number of recent sets of guidelines now advise the use of NIR spectroscopy as a universal method suitable for the identification of raw materials:
- European Pharmacopoeia 5 (2005), page 59;
- EMEA CPMP/QWP/3309/01 and EMEA/CVMP/961/ 01 Note for Guidance on the use of Near infrared spectroscopy by the pharmaceutical industry and the data requirements for new submissions and variations, EMEA, London, 2003;
- USP 29 (2006), page 2979.
NIR Spectroscopy
EMEA, Note for Guidance
The identification of a substance with NIR spectroscopy is based on a comparison between the spectral data of the substance being analyzed and the spectral data of multiple samples of batches in a reference library. To compare the data and come to conclusions, chemometrics should be used.
For the characterization of raw materials, the technique has been found to have some unique benefits. NIR spectroscopy enables analysis of the starting material in the original packaging, without the need to open the main container, thus reducing the risk of cross-contamination and abolishing the need to conduct the analysis within a designated sampling area. This leads to a significant decrease in the time involved for each analysis. For example, the time required for identification of one container of raw material using IR spectroscopy is approximately 15 minutes, using color reaction and viscosity is approximately 45 minutes, while identification of the same amount using NIR spectroscopy takes approximately two minutes.
NIR spectroscopy can be used to collect high-quality reflectance spectra of both the active ingredient and excipients, and is sensitive to both the chemical and physical properties of the powder blend. It can also be beneficial within pharmaceutical manufacturing as it is non-contact and non-destructive, highly reproducible, rapid and does not require any sample preparation. Online blend monitoring has placed certain demands on NIR instrumentation including wireless communication, battery operation, rapid data collection, appropriate hazard and cleaning rating and software/hardware validation and qualification. It is also important that the NIR instrumentation can be used from pilot to production scale blenders in order to follow the development of a pharmaceutical product.
Within a pharmaceutical manufacturing plant, NIR spectroscopy can be carried out at multiple stages in the manufacturing process: in the raw materials warehouse, in the dispensing area or in the QC laboratory. Considerations need to be given to the individual company and its requirements. If the analysis is carried out in the warehouse, no sampling is required by GMP guidelines and therefore the time for analysis is reduced.
There are also practical advantages, as raw materials can be analyzed as they arrive in the warehouse, and do not have to be transported for analysis, ensuring improved workflow. However, placing the analyzer in the raw materials warehouse means that the analysis may not be carried out by specialized personnel. This can be remedied by using correct training for staff, and by appointing an instrument manager to oversee the raw material identification.
Figure 1: NIR spectroscopy can be conducted at multiple stages in the manufacturing process, including in the raw materials warehouse (shown: Abiogen).
If the analysis takes place in the dispensing area, there is again no need for sampling, which results in a reduction in analysis time. There are also cost savings on reagents and other chemicals, however the analysis is again carried out by non-specialized personnel and the flow of raw materials through the manufacturing plant is interrupted. A further option is to place the analyzer in the QC laboratory, where specialized personnel can carry out NIR spectroscopy analysis. Although time per analysis would be reduced, in order to comply with GMP guidelines, it would be necessary to sample, also again interrupt the flow of raw materials. When choosing to use NIR spectroscopy, each individual company must decide where best to place the analyzer.
Example
Abiogen Pharma S.p.A is a pharmaceutical manufacturer based in Pisa, Italy. Abiogen manufactures its own products and carries out contract manufacturing for the pharmaceutical sector, including products such as granules, filmed tablets, pills, capsules, ointment, lyophilized products, syrups, drops, suppositories and aseptic ampoules. The main areas of Abiogens business are R&D and manufacturing of pharmaceutical products, and the company has laboratories dealing with QC (chemical and microbiological), pharmaceutical development (analytical development, QC of medicinal products and formulation) and research laboratories. Abiogen develops pharmaceutical products mainly for the musculoskeletal, respiratory, diabetology and dermatology areas.
Results
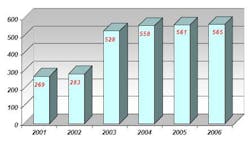
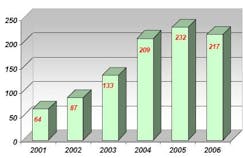
Prior to using NIR spectroscopy for raw material analysis, Abiogen used individual methods for each raw material container in accordance with the European Pharmacopoeia guidelines, including UV-Vis spectroscopy, infrared spectroscopy and gas chromatography (GC). Conducting raw material identification with these methods proved to be extremely time consuming. Additionally, the amount of incoming raw materials doubled from 2001 to 2006 (see Table 2), while the number of differing types of raw materials arriving at Abiogen tripled in this period (see Table 3).
Table 2: Amount of incoming raw materials to Abiogen, 2001-2006
Table 3: Number of different incoming raw materials to Abiogen, 2001-2006
To continue sampling each different raw material individually, it would have been necessary to engage a second sampler to cope with the workload. This would cause an increase in cost, in addition to the increase in time consumed by the growing number of samples to analyze. Carrying out separate analyses for each raw material also takes a huge amount of planning and organization, taking scientists away from the laboratory and increasing their workload.
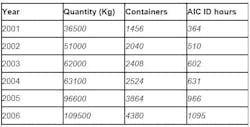
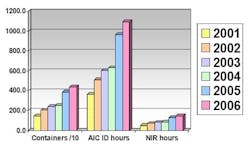
One particular raw material that was greatly increasing in quantity was Metformine Hydrochloride. At a standard weight of 25 kg per container, this raw material was originally analyzed using IR spectroscopy. This method proved no longer viable when the number of containers of Metformine Hydrochloride began to significantly increase (see Table 4), resulting in an increasing number of hours required for material identification.
Table 4: Increase in container quantity and analysis time of metformine hydrochloride, 2001-2006
Figure 2: Metfonorm: Abiogens diabetology product (metformine hydrochloride active ingredient).
Abiogen examined alternative methods of analysis for metformine hydrochloride, including increased container sizes and material self-qualification from suppliers. Increasing container sizes was not asuitable option, as it can be implemented only for low-cost raw materials, and would have had a significant impact on manufacturing processes such as transport and weighing systems. Although GMP allows a lower number of tests on raw materials supplied by qualified vendors, this was not a suitable alternative for Abiogen as the implementation process can be lengthy, and there is a periodic vendor re-qualification requirement which could result in the necessity to change suppliers.Figure 3: Thermo Scientific Antaris FT-NIR analyzer in Abiogens raw materials warehouse.
Having considered these alternatives, Abiogen chose to implement NIR spectroscopy with a Thermo Scientific Antaris FT-NIR analyzer in order to save time and costs and to increase productivity. Abiogen made the decision to place the FT-NIR analyzer in the raw materials warehouse, in order to comply with GMP guidelines while maintaining a reduction in the time per analysis and the operating costs of the analyzer.
Since the implementation of NIR spectroscopy, Abiogen has significantly increased productivity due to no longer having to sample each container of raw material (see Table 5), in addition to saving time per analysis and reducing instrument and chemical costs.
Table 5: Comparison of analysis time using IR spectroscopy and NIR spectroscopy
Table 5 shows the increase in the number of containers of raw materials (containers/10), as well as the increase in the number of hours spent using the AIC-recommended IR spectroscopy analysis. It is evident that a significant time reduction resulted from using NIR spectroscopy, compared with traditional methods used.
Conclusion
NIR spectroscopy can be an extremely accurate and beneficial method for the analysis and QC of raw materials in the pharmaceutical manufacturing plant. In addition to helping laboratory and manufacturing workers to improve productivity by reducing analysis time, using NIR spectroscopy can also aid regulatory compliance. In recent years NIR spectroscopy has been recommended as a valuable tool for raw material analysis by a range of pharmaceutical guidelines, including the Pharmaceutical Analytics Science Groups guidelines for the developments and validation of NIR spectroscopic methods, and the European Agency for the Evaluation of Medical Products note for guidance on the use of NIR spectroscopy by the pharmaceutical industry. It is also possible to see by looking at the practical example of Abiogen, that using NIR spectroscopy for raw material analysis can save a significant amount of time in the laboratory, as well as improving productivity, and reducing operating costs.
References
- Chapter 5.30, GMP guidelines
- Annex 8, EU.GMP
- EMEA, Note for Guidance on the Use of NIR Spectroscopy by the Pharmaceutical Industry and the Data Requirements for New Submissions and Variations
About Abiogen
Abiogen Pharma S.p.A., is a pharmaceutical manufacturer based in Pisa, Italy. Managed by Massimo Di Martino, the company has over 370 employees and an annual turnover of 69 million.The company is subdivided into three strategic business areas: R&D (Research & Development), Manufacturing (production of pharmaceutical specialities) and Pharma (Sales of Pharmaceutical Specialities). In 2006 Abiogen invested nearly 6.3 million in research with the aim to launch a range of new products.
About Thermo Fisher Scientific
Serving customers through two premier brands, Thermo Scientific and Fisher Scientific, Thermo Fisher Scientific Inc. helps solve analytical challenges from routine testing to complex research and discovery. Thermo Scientific offers customers a complete range of high-end analytical instruments as well as laboratory equipment, software, services, consumables and reagents to enable integrated laboratory workflow solutions. Fisher Scientific provides a complete portfolio of laboratory equipment, chemicals, supplies and services used in healthcare, scientific research, safety and education.
For more information on the Thermo Scientific Antaris FT-NIR analyzer, please call +1 800-532-4752, email [email protected] or visit www.thermo.com/NIR.