Today, a growing number of pharmaceutical manufacturers are using advanced aseptic processing technologies to minimize operator intervention and contamination risk in the filling and packaging of liquid parenteral drugs. One of these technologies is form-fill-seal (FFS), in which a polymeric material is formed and sealed inline to a container of choice, while the container is being filled.
FFS offers cost savings over conventional aseptic processing in glass. Traditional parenteral filling and packaging requires 23 steps and individual machines for filling, stoppering and capping. In contrast, FFS requires one piece of automated machinery, and takes place in six seconds or less.
The entire FFS process is performed under a class-100 laminar flow, preventing external contamination. The fully automatic, computer-controlled technology allows for filling and packaging of up to 40,000 bottles of IV fluid per day. Nitrogen purging options are available for sensitive formulations such as amino acids.
Sterilization is achieved through an automatic, microprocessor controlled, circulating water-shower. The water becomes sterile during the process without any hazard to the product. The pressure/temperature link controls the whole process. The system uses a nylon filter medium to remove colloidal silica, pyrogens, mycoplasma, viruses and other contaminants.
A typical FFS process works as follows.
- First, bulk solution prepared under aseptic conditions (as appropriate) is delivered to the machine through a bacteria-retaining filter. Pipework, filter housings and machine parts that are in contact with the product are steam sterilized in place.
- Filtered compressed air and granules of a plastic material conforming to a predetermined specification and known to be compatible with the product to be filled (usually polyethylene, polypropylene or polyethylene/polypropylene co-polymers) are supplied to the machine.
- Within the machine, the plastic granules are extruded downwards under pressure (up to 350 bar) as a hot hollow moldable plastics tube (or parison) or tubes. As a result of the high pressure extrusion process, the parison reaches a temperature of 170° - 230° C. The configuration and internal integrity of the parison are maintained by an internal downward flow of filtered air under pressure.
- The two halves of a mold close around the parison to seal the base. Simultaneously, the top of the parison is cut free by a hot knife-edge. The plastics material is now formed into a container(s) by vacuum and/or sterile air pressure.
- The container(s) is/are immediately filled with a metered volume of the solution, displacing the sterile air. Both the air and the solution are filtered through bacteria-retaining filters immediately before entry into the forming, or formed container(s).
- When the required volume is filled into the container(s), the filling unit is raised and the containers are sealed automatically. The mold then opens, releasing a package formed, filled and sealed in one continuous, automatic cycle. Meanwhile, parison-extrusion continues, and the cycle repeats. The filled and sealed units usually require some cropping of excess plastic.
When used for aseptic manufacturing, the cycle is conducted automatically within the machines own internal sterile air flushed environment (or air shower). The range, accuracy, reproducibility and response time of all controlling and recording instruments associated with the FFS machine and all supporting equipment, must be adequate to ensure that defined process conditions will be consistent during routine production. All instruments must be calibrated before any meaningful operational qualification can be performed. Written calibration procedures should specify the methods to be used for each instrument. Recalibration should be carried out after any maintenance, and all records maintained. New machine specs should state requirements for:
Materials of construction for all components, particularly all contact parts, such as machine pipe work; internal components of purchased fittings like automatic valves including elastomeric and mechanical seals; pipeline joint seals; welding materials; filters and filter housings including casing and substrate layers of cartridges, as well as the main medium and all elastomeric seals; and polymer extrusion equipment.
Pipe work configuration, with attention to sterile fluid pathways for example, the elimination of deadlegs; position of thermocouples (as installed configuration, verified against the original design configuration and confirmed by temperature mapping is typically part of the validation protocol); and filter housing design.
Porosity of the product and air filters. The validation data from the filter manufacturers should be available.
Mold design, considering fill volume range, wall thickness, opening characteristics and ease of use, shape and other aesthetic considerations.
If FFS machines are used for the manufacture of non-sterile products, FDAs current Good Manufacturing Practices (cGMP) requirements should be followed. When used to manufacture products intended for subsequent sterilization, these machines may be installed within an environment that would normally be considered appropriate for the manufacture and filling of terminally sterilized products. If the machines are to be used for the aseptic filling of sterile products they are usually provided with a localized environment at the point of fill with Grade A air.
The Installation Qualification process for any FFS system should confirm and certify that the room conforms to the specified Environmental Standard. A new cleanroom installation should include: room air filter integrity tests; determination of air velocity at the face of each air inlet filter; room air change rate; air particle counts, both viable and non-viable, in the rest condition; room pressure differentials; and lighting, heating and humidity readings.
Following the initial commissioning, a regular re-test program should be adopted. Some of these tests include:
Room Air Filter Test at least once a year
Air Velocity twice a year.
Air Particle Counts: Determine as part of regular in-process monitoring with formal certification by a competent specialist agency twice a year.
Room pressure differentials should be monitored on an ongoing basis. Walls, floors and surfaces should be subject to a pre-determined program of cleaning and disinfection.
A complete, on-going maintenance program should be developed and implemented. Matters to be specifically covered in the maintenance program should include those items listed under Equipment Qualification. In addition, examination and replacement of elastomeric seals, and the condition of molds, dies and pins should be monitored. The program applies to all supporting equipment and instruments as well. An in-process control and monitoring program is necessary for environmental particulates, filter integrity, microbiological concerns and product control. The environmental air should be checked so that it remains in conformity with the specification. The immediate air shower environment also should conform to specifications during processing with respect to viable and, where possible, nonviable particulate matter.
Filter integrity testing is needed for the filter(s) that are used to sterilize the product, and the filter(s) used to ensure the required air quality within the FFS machines. The internal air shower and container molding process is critical in sterile product manufacturing. Filter integrity tests of the product filter must be conducted after each and every use of the filters. It is recommended that filter integrity testing be performed before the filtration of the product commences and after the batch, or lot, has been filtered.
The following points should be considered for microbiological monitoring and control procedures:
Bioburden check on bulk solution, before delivery to the FFS machine.
Exposure of settle plates (petri dishes of nutrient agar) at critical positions within the general room where the machine is sited.
Use of air sampling devices to determine the number of viable organisms per cubic foot of air in the room.
Use of contact plates and swabs to see the microbiological quality of surfaces. Once filling is finished, operator entry into the machine room should be kept to a minimum. Operator finger dabs provide an additional microbiological control.
In-process, the product should be continuously checked for appearance, fill volume, wall-thickness of container, container leaks and container opening characteristics. This entire in-process monitoring program should be conducted as per the schedule and written specified test limits and standards. All results should be reported and evaluated formally against those limits.
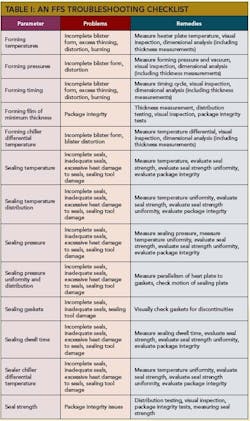
The Table below lists the major process parameters and their risk to package integrity. All of these parameters affect the packaging process.
Regulatory Issues
The regulatory guidance recommends that FFS machinery and its surrounding barriers be designed to prevent the potential for extraneous contamination. In addition, a validated steam-in-place cycle or equivalent process should be used to sterilize the equipment path through which the product is conveyed.
The guidance also notes that the classified environment surrounding form-fill-seal machinery should generally meet Class 100,000 (ISO 8) or better. HEPA-filtered or sterile air provided by membrane filters should also be used when sterile products or materials are exposed. Air in the critical area should meet Class 100 (ISO 5) microbiological standards during operations. Finally, a well-designed system should achieve Class 100 (ISO 5) airborne particle levels.
Limitations of FFS
Volatility and viscosity are two limiting factors of FFS technology. If the solvent is volatile in nature (e.g., alcohol, if alcohol content is more than 5%), then FFS technology is not appropriate. However, a FFS machine with an explosion-proof design can fill solutions with alcohol content up to 95%. Viscosity also can be an obstacle. Slight warming may temporarily reduce viscosity enough to allow filling.
References
1. Agalloco, J., Akers, J and Madsen, R., What is Advanced Aseptic Processing?, Pharmaceutical Manufacturing, February 2006, p. 25.
2. Berrebi, H., The bottle-pack system for the pharmaceutical industry, pub. Rommelag AG, Hintere Bahnhofstrasse 78, CH-5001, Aarau, Switzerland.
3. Leo, F., Chapter in Aseptic Pharmaceutical Manufacturing - Technology for the 1990s. Ed. Olson and Groves. pub. Interpharm Press.
4. Sharp, J.R., Manufacture of sterile pharmaceutical products using blow-fill-seal technology, Pharmaceutical Journal, 1987, 239, 106.
5. Sharp, J.R., Validation of a new form-fill-seal installation, Manufacturing Chemist, Feb. 1988, p.22.
6. Zimmerman, L., Technical measures for aseptic packaging of liquids with the bottlepack-asepticsystem.
About the Authors
All authors are affiliated with the S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Kherva, Gujarat, India.
Rishad Jivani is doing his post-graduate education in Pharmaceutics.