FMEA: A Risk-Based Approach to Sterility Assurance
Defuse Quality Problems Before They HappenEditor's Note: Product recalls, consent decrees, 483s, lost batches. We all know what happens when drug manufacturers lose control of a critical quality parameter, or fail to estimate infrastructure needs. Experts agree that data monitoring, trending and analysis are all that is needed. But thats often easier said than done. Deciding which data points are the most critical to product safety and quality can be challenging. Proactive manufacturers prioritize efforts effectively, using various methods and technologies to focus on areas that will yield the greatest return, have the greatest impact on product quality and safety, and optimize process control. The following are case studies in proactivity, approaching the topic from different angles.
As these articles show, optimizing quality proactively is not only possible, but is becoming an established practice for more pharmaceutical manufacturing operations. Is yours among them? |
Demonstrating a high degree of sterility assurance is critical to successful aseptic processing. Each process step and each component introduces opportunities for error that can lead to a loss of sterility assurance. The variety of utility systems, lab systems and clean room systems involved in the process further add to the complexity. Proper validation and control of the manufacturing system is crucial since the product may not be suitable for terminal sterilization in its final packaging.
This article describes a proactive method of improving sterility assurance using a risk-based approach. This initiative focused on the use of Failure Mode and Effects Analysis (FMEA) to identify potential weak points in the sterility management process.
Gathering Data
The manufacturing facility described here is still early in its life cycle. Thus far, the sterility program has been solid, with no failures and few questions about the assurance of sterility. Despite this good performance, a proactive approach was taken to identify potential failures in our system. In the spirit of continuous improvement, we thought it was important to leverage our good experiences in sterility management as we sought to improve our existing process.
Typically, an FMEA would start with a detailed review of historical failure data. This step is important to remove opinion and conjecture from the analysis. However, with limited historical data on loss of sterility assurance, we sought information from process experts to help identify potential areas of concern. The first step was to interview a number of subject-matter experts across our corporation, including personnel in quality assurance, validation, manufacturing and the QC labs.
Next, our team sought ideas from operations personnel across the site. Groups from each of the aseptic manufacturing areas, the utilities area and microbiology were consulted. Brainstorming sessions with each of these groups across several shifts were organized.
Our team was concerned that traditional verbal brainstorming might inhibit responses from some individuals who might be uncomfortable in a group. There also was a concern that more dominant individuals would take over the conversation and thus we would miss some good information from quieter personnel. Therefore, our team made use of an alternative brainstorming method called Brainwriting 6-3-5.
This technique is a written brainstorming tool that refers to having six people each write down three responses to a problem in five minutes. Each participant begins with a worksheet divided into five rows of three columns. A question or problem statement is listed at the top of the page. Each person records three responses to the problem or question within five minutes. Each worksheet is then passed to the left and a new five-minute period begins. Each person reads the first three responses and then writes down three more ideas until the table is full.
Our team developed several questions related to sterility assurance. Some typical questions were:
- What is the toughest task for you to perform aseptically?
- What activities have you seen that cause you concern for sterility assurance?
- What do you focus on most for sterility? Why?
We then began each group session by working through a couple of the Brainwriting exercises. We varied the number of rounds and the time to respond based on the make-up of the group in each session. After completing the written brainstorming event, we moved into a more traditional verbal brainstorming session. This session was an open forum for presenting concerns about sterility as well as to share best practices among the team.
This combined approach to idea generation led to tremendous results. The written responses were thoughtful and detailed. The Brainwriting exercise then seeded the verbal brainstorming session. The verbal brainstorming was successful because each individual had the time to put his or her thoughts together during the written session. Using this combined approach, our team was able to gather over 200 potential sterility concerns.
Failure Mode and Effects Analysis
The Sterility Assurance Improvement Team then took the ideas generated by the small group sessions and broke them down into categories. Each line item was categorized as either Equipment-, Material-, People- or Method-related. This categorization made it easier to break the list down into smaller sections for the FMEA.
Failure Mode and Effects Analysis is a tool for risk analysis that has been used for many years. First utilized in the safety area, it has since expanded into a quality improvement tool. An FMEA can be either based on actual historical failure data or on potential failure modes. For an aseptic process, a failure can be defined as a loss of sterility assurance. This does not necessarily mean that the product is non-sterile. It simply means that you have lost the assurance of demonstrating sterility.
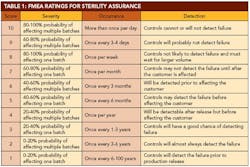
After identifying all the potential failure modes, the next exercise was to identify the effects of this failure. Using a 1-to-10 scale, the team identified the severity of each effect, with 10 being the most severe. The severity rating was applied without regard to frequency of occurrence or any controls that might be in place. The rating is simply an indication of the effect of the failure, should it occur. The team then listed the potential causes of each effect and assigned an occurrence number, with 10 being the most frequent cause.
The best case for an FMEA is to use historical failure data to populate the occurrence values. Since very limited data were available on loss of sterility assurance, we compensated by gathering ideas from personnel with a broad cross-section of experiences. The team also identified any controls in place to detect and/or mitigate an impending failure. This value is an indication of the probability of detecting a failure before it impacts the customer. A detection number assigned with 10 represents the lowest likelihood of detecting the failure.
A 10-person FMEA team was created, including subject-matter experts in various aspects of aseptic processing. Personnel were included from operations, validation, microbiology and other related groups. The team agreed on qualitative descriptions for each of the values for severity, occurrence and detection. It is important to agree on these values before evaluating each line item. This allows a fair evaluation of each item as a stand-alone idea. The agreed-upon definitions are detailed in Table 1 (below).
Since a loss of confidence in sterility is always unacceptable, it would be difficult to have 10 separate ratings for severity. A loss of sterility assurance would always receive a rating of 10. Therefore, the team focused on the probability of affecting sterility in one or multiple batches. An increasing probability of affecting one or more batches led to an increasingly higher severity value. Items that could potentially affect multiple batches, as opposed to an isolated event that only affected one batch, received higher severity ratings as well.
The FMEA team worked through each line item in the FMEA template, and assigned a value for severity, occurrence and detection. These three numbers were then multiplied together for each line item to get a Risk Priority Number (RPN). A higher RPN number represents a greater risk of achieving failure, or losing sterility assurance. The RPN numbers were then analyzed to create a prioritized list of action items to reduce the risk of failure.
Using the criteria in Table 1, the FMEA team met several times to evaluate each line item generated during the brainstorming sessions. For each line item, the team reviewed the potential effects of a failure. This led to the assignment of a severity rating based on process knowledge. The team then reviewed potential causes that could lead to the failure. Historical data were reviewed and team experience was considered to assign an occurrence rating. Finally, the team considered all automatic and manual controls that were in place to detect failure or impending failure. A detection rating was assigned based on Table 1 criteria.
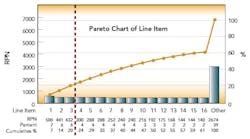
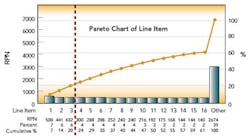
After this process, the Risk Priority Number for each FMEA line item was calculated and ranked by RPN. The first level of analysis was to create a dot plot of the RPN values from the table. The RPN numbers are mainly clustered at low RPN values. This is an indication that there are very few critical items that need to be addressed. This was expected, based on the good history of sterility management at the site. The RPN numbers begin to separate from the cluster at values above 140.
These 15 items warrant attention. The three highest RPN values are clearly separated from the rest of the line items. As the Pareto diagram (above) shows, these three RPN values account for 20% of the total summed RPN values of all line items. Using the Pareto principle, we would expect these top 20% of our total RPN to contain 80% of the risk. Therefore, these three items should be the focus of immediate proactive actions resulting from the FMEA exercise.
The RPN numbers generated by the FMEA steer the path forward. The top three items have been referred to each affected area for immediate attention. The rest of the top 15 items have been presented to the site Operational Quality Team and the affected area personnel. Each of these items will be further investigated to develop appropriate actions to reduce the product sterility risk.
Suggested Reading
- McDermott. R., Mikulak, R., et al., The Basics of FMEA, Productivity, Inc., Portland, Ore., 1996.
- Ritter, D. and Brassard, M., The Creativity Tools Memory Jogger, GOAL/QPC, Salem, N.H., 1998.
- The Black Belt Memory Jogger, Six Sigma Academy, GOAL/QPC, Salem, N.H., 2002.
- Brassard, M. et al., The Six Sigma Memory Jogger II, GOAL/QPC, Salem, N.H., 1994.
About the Author
Craig Alexander, P.E., is a Plant Improvement Engineer and Manufacturing Technologist with Monsanto in Augusta, Ga. He has been with Monsanto for 15 years with various engineering responsibilities including instrumentation, automation, process optimization, and simulation. Craig holds a Bachelor of Electrical Engineering degree from Auburn University and a Master of Science in Engineering degree from the University of New Orleans. A registered P.E. in Georgia and Louisiana, he is a member of ISA and ISPE.