Japanese pharma is entering the brave new world of personalized medicine, and the BIO show in Chicago in April brought with it an innovative company that is exploring new cancer treatments. Established three years ago, Green Peptide Co., Ltd. (Fukuoka, Japan) is developing targeted immunotherapies for malignant brain tumors and prostate cancer. Last August and November, the company submitted IND applications for Phase I studies in Japan.
Named for the fact that its therapies work naturally, with the bodys own processes, Green Peptides main focus right now is cancer treatment, and providing patients with a better quality of life alternative to chemotherapy or radiation therapy, explains Dr. Keizo Yoshida, R&D director. Tests conducted at the Universitys hospital, and reported in Clinical Cancer Research in 2005 showed that the immune treatment improved survival and had fewer adverse effects on patients than treatment alternatives.
The basic mechanism behind Green Peptides therapies would also allow Green Peptide to develop other treatments for pancreatic, stomach, colorectal and other forms of cancer, as well as infectious diseases such as hepatitis C, and even allergies such as hay fever.
Last year, Green Peptide established a sister company, ImmunoDia, to focus on diagnostics related to these therapies; this company would conduct studies in patients to determine those who would be most likely to respond to treatment, offering patients an alternative to more invasive treatments.
The foundation for both companies is research that began around 1992 at Kurumes School of Medicines immunology department. University researchers isolated over 100 genes that encode for cancer antigens, identifying more than 200 antigenic peptides. Japan, unlike the U.S., allows some early clinical testing on novel compounds; six years after research had started, early clinical research at the university began, ultimately confirming the safety and efficacy of these peptides for use in vaccines, Dr. Yoshida says.
Green Peptides therapeutic peptides bind with the histocompatibility antigen (HLA molecule) on the surface of antigen-presenting cells, promoting destruction of the cells by T lymphocytes within the bodys immune system.
The treatments wont work for everyone. Once basic blood tests identify a potential responder, ImmunoDia would use various immunodiagnosis techniques (antibody, CTL and HLA) to determine the best peptides to use for that patient. The peptides would then administered by injection, and the patients response monitored.
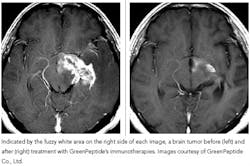
Clinical studies on 400 cancer patients found that a select group of the peptides was very effective in treating some cancers, and that the same set of peptides could be used to treat different cancers. The most dramatic results were observed in the treatment of malignant brain tumors, where the peptides were observed to cause dramatic shrinkage within six months (see photo below). Results were also evident in treating hormone-refractory prostate cancer, where treatments appeared to stop bone metastasis in some patients. Hepatitis C therapies are at the translational research phase at the university right now.
The company has applied to patents for its prostate cancer and hepatitis C peptides, as well as the method it uses to select those peptides, in the U.S., Europe and Asia, and is exploring several different types of business models with potential partners, ranging from marketing to cooperative development and licensing agreements.
Green Peptide is currently working with NeoMPS, Inc. (San Francisco, Calif.) and American Peptide Co. (Sunnyvale, Calif.) to manufacture the peptide drug substance for clinical trials, and with Toyobo Co. Ltd. (Osaka) on product formulation.