Achieving Process Understanding: The Foundation of a Strategic PAT Program
Imagine a group of pharmaceutical manufacturing experts who convene to discuss the FDA’s Process Analytical Technology (PAT) initiative and over the course of two days never once delve into near infrared spectroscopy (NIR) — or virtually any other on-line technology. That’s exactly what happened at a recent PAT symposium comprising pharmaceutical executives, industry consultants, academics, and one of the initial leaders of the FDA’s PAT effort, Ajaz Hussain. Far from being a glaring omission, however, the relative absence of such discussion provided a striking confirmation of the real opportunity that PAT offers pharmaceutical companies: a chance to achieve systematic improvement in manufacturability through application of a holistic, strategic implementation framework designed to generate significant business benefits.
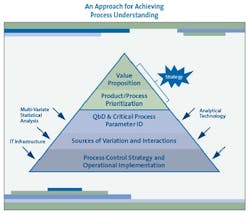
As Figure 1 (below) suggests, the most effective PAT programs begin by putting PAT in a strategic context, including an appreciation of the value it can generate and a prioritization of the products and processes to which PAT can be applied to realize that value. Nevertheless, few companies launch their PAT initiatives in this organized and logical fashion. Most companies “doing PAT” today continue to be focused on the search for applications of technology such as NIR.
After establishing the PAT value proposition and prioritizing products and processes, attention should turn next to improving process understanding for those products and processes where there is value to be gained. The three elements for achieving that all-important process understanding include:
- Utilizing
- quality by design (QbD) techniques and identifying critical process parameters (CPPs)
- Identifying
- drivers of variation and of interactions in critical processes
- Implementing
- process control strategies.
Again, as Figure 1 depicts, analytical technology is not the central focus of the effort, but an enabler of the real goal: the understanding, variation reduction, and control of critical process parameters in the business context of maximizing value.
Understanding the Value
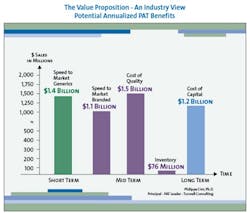
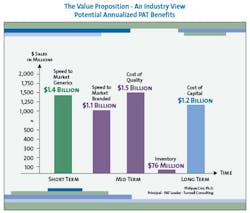
The value to be gained from improved manufacturing through superior process understanding is enormous. Although the scale and precise distribution of savings will differ for each pharmaceutical company, a strategic PAT program can yield millions of dollars in savings in four key areas: reduced cost of quality, reduced capital investment, reduced inventory, and increased speed to market (see Figure 2, below).
In the pharmaceutical industry the first pass defect rate, which is an expression of lack of process understanding, is estimated to be approximately 15% (2.5 sigma). For that reason, the largest contributor to overall savings arising from an extensive PAT program will generally be reduced cost of quality. Fortunately, inspection regimens prevent these first-pass defects from reaching the marketplace, increasing quality to 5.5 sigma or 0.003% defects; however, improved process understanding can greatly reduce those defects in the first place and thereby greatly lower the cost of quality.
It is currently estimated that for most pharmaceutical companies, the total cost of quality is, on average, about 25% of sales, with much of that cost resulting from poor process understanding, which a PAT initiative could dramatically reduce. In fact, one estimate puts the potential savings from PAT-driven reduced cost of quality at over $1 billion for each of the top 10 pharma companies.
Similarly, savings from reduced capital investment can also be significant. Consider that for each of the top ten pharmaceutical companies, 2005 capital expenditure (CAPEX) is forecasted to be 11% of sales on average, or about $3.2 billion. (Interestingly, in 2004, CAPEX spending was only 5.3% of sales.) PAT technology could become a key enabler to move from batch to continuous manufacturing, in which materials are modified and tested continuously to minimize delays in movement from start to finish during the process. Assuming no change in production volumes, continuous manufacturing requires significantly less square footage and equipment.
Continuous manufacturing would therefore enable companies to manufacture the same amount of product in smaller factories, or manufacture far more product in existing facilities. Because CAPEX is correlated with square footage, CAPEX would also be expected to go down. For each of the top ten pharmaceutical companies a 50% reduction in CAPEX would translate into an average savings range of $0.8 to $1.6 billion per year.
On-line and at-line testing could also reduce production cycle time. Reduced production cycle times would, in turn, decrease inventory in the system and save inventory carrying costs, which average $76 million for each of the top ten pharmaceutical companies. In addition, breakthrough improvements in drug development that improved process understanding can greatly reduce time to market, resulting in far greater return on investment through longer patent protection and first-to-market advantage.
Prioritizing of Products and Processes
To help realize the maximum business value from PAT, your program must be cost-effectively deployed where it will do the most good to improve quality, consistency, throughput, and speed to market. The focus might be to optimize a particular unit operation across product lines or to optimize a particular product across all its unit operations. In general, this author favors the latter strategy as it will eliminate the chance that in an effort to optimize one unit operation the total process becomes sub-optimized.
In prioritizing products for PAT, you should also consider products in manufacturing as well as in development. The PAT initiative initially appeared to focus on applications in drug development, where PAT can maximize the probability that a potential product will make it to validation, scale-up, and technology transfer with minimal glitches. But the FDA has made it clear that it is also encouraging the use of PAT strategies for the manufacture of currently approved products.
In both instances, the focus should be on value. For products already in manufacturing, optimizing current processes can lower costs, increase productivity, and lower compliance risk. During the prioritization process, allocating PAT resources between products in development and products in manufacturing offers the opportunity to balance short-term and long-term value contribution in your company’s product portfolio.
Achieving Process Understanding
Achieving process understanding begins with an identification and understanding of critical process parameters (CPPs) — those “few” process parameters that must be maintained within critical limits to achieve the desired quality outcome. In many pharmaceutical companies today, efforts to understand processes often focus on application of analytical technology to surrogate measures for batch release parameters (e.g., coating thickness) or on operating conditions that are predictive of good batch release parameter results (e.g., blend uniformity). But these efforts typically fail to determine if what is being measured is indeed critical to controlling end product variation and virtually never improves understanding of the interactions between parameters.
Similarly, batch release testing alone does not yield the same degree of certainty that release testing in conjunction with process understanding provides. Batch release testing assumes a Gaussian, or normal, distribution of batch characteristics. Moreover, it assumes that variability from dosage unit to dosage unit is relatively small, allowing a modest sample size. However, these are not always sound assumptions. Improvements in process understanding at the unit operation level can significantly improve the confidence we have that batches passing release testing are truly “good” batches.
So how do you know when you understand your processes? When:
- The impact of changes in critical process inputs can be predicted, thereby anticipating process control problems.
- All critical sources of variability (and interactions) are identified and explained.
- Product specifications are based on understanding of drivers of variation and process capability.
Quality by Design
The optimum approach to achieving genuine process understanding will depend on whether the process under investigation is associated with a product that is being developed or has been developed using quality by design (QbD) or a product that was developed using traditional methods.
“Quality by design,” a term borrowed from quality pioneer Joseph Juran, is intended by the FDA to mean that product and process performance characteristics are scientifically designed to meet specific objectives, not merely empirically derived from performance of test batches. During QbD development of a product, the development team seeks to establish the product’s “design space” — the multi-variate relationships that encompass combinations of product design, manufacturing process design, manufacturing process parameters, and raw material quality that provide assurance of suitable quality and performance.
First, through a combination of prior knowledge and experimental assessment during development, you identify the characteristics or operating conditions under which a product is going to be manufactured and seek to understand which ones are important. From this knowledge and data, a multivariate model linking product and process measurements and desired attributes may be constructed, which enables you to accurately and reliably predict product quality attributes over the design space.
|
|
For example, for a time-release solid dose product, you might decide to explore a design space for a coating spray rate of 500-1000 gm/min. Of course, the same would be done for each operating parameter being studied. The relationships between all of these parameters are then studied with a focus on understanding potential interactions.
Once you understand the design space, then you can set specifications, which are a subset of that space. In the example of the coating spray rate, which you have explored in a range from 500-1000 gm/min, you might ultimately set the specifications from 700 to 800 gm/min because you found a “sweet spot” using statistical predictive modeling (discussed here later) that results in optimum product quality or performance. In this scenario, process specifications are set based on an understanding of drivers of variation, interactions, and process capability.
QbD also dramatically changes the approach to quality once the drug is in production. As all pharmaceutical manufacturers know, once a drug is in production it can “drift.” Ordinarily, that has meant time- and resource-intensive investigations — often on a univariate basis — for the causes of these deviations. In some instances, the solution to the problem requires a change to the manufacturing process or to normal operating ranges. In both cases there are regulatory implications. However, for a product developed under a QbD regime, it is not only easier to model the deviations and find the source of the variation but also to minimize the level of effort associated with the required regulatory action (e.g. Annual reportable change vs. CBE 30 vs. Prior Approval Supplement).
Again, consider the example of the coating spray rate explored from 500-1000 gm/min and set at 700-800 gm/min during development. Suppose that after launch of the product you find that a better spray rate is 600-700 gm/min. Because the design space you have already explored encompasses this lower spray rate and you have firmly established process understanding in this range, it will likely reduce the required regulatory effort to implement this change.
Unfortunately, most companies have not approached development as an opportunity to explore processes fully and firmly establish process understanding for broad ranges of multi-variate conditions. Instead, they try to establish a range of acceptable specifications as quickly as possible and hope that nothing changes once the drug goes into production. But they are often only putting off the inevitable.
When things change, as they so often do after launch, whatever time and cost savings were gained by quickly speccing the product in development are soon dissipated when quality and performance problems arise in production. But with QbD, you can define the design space broadly enough to know where the safe harbors are when conditions change, quickly rectify the problem, and potentially avoid time-consuming investigations and regulatory action. Thus, QbD not only eases the regulatory burden but also fosters a culture of continuous improvement by eliminating the fear of looking too deeply into processes.
Process Understanding for Non-QbD Legacy Processes
Many drugs already in production, of course, were not developed under QbD conditions, and processes drift out of specifications for a variety of reasons — changes in API, changes in excipients, changes in process equipment, to name a few. Many non-QbD products are developed without sufficient process characterization before validation, and some of the CPPs haven’t been identified and few of the parameter interactions are understood. Moreover, the standard three validation batches are insufficient for understanding the combined effects of multiple CPPs, even when all of the parameters are controlled within the ranges supported by development and specified in the validation protocol and batch record.
The FDA’s PAT initiative, which recognizes that the current regulatory system has been unfavorable to the introduction of innovative systems, now encourages manufacturers to continuously improve and optimize their processes. Faced with post-manufacturing problems, manufacturers are increasingly using a variety of proven statistical techniques to identify critical process parameters, drive toward an understanding of sources of variations and interactions, optimize their processes, and avoid onerous compliance and regulatory burdens.
Tunnell Consulting has been using a structured approach to improving and optimizing pharmaceutical manufacturing processes for many years. The approach begins with development of qualitative process understanding. Through focus interviews, process maps, and document review, the improvement team can begin to develop and refine hypotheses about the possible sources of sub-optimal process performance.
The next step is to perform some basic quantitative characterization of the process, using statistical tools such as control charts and Cpks. Control charts help identify process data patterns that indicate a potential problem. Shifts, trends, or outliers can be correlated with a change in the process or a raw material, providing insight into the root cause of the problem.
Cpk provides a quantitative assessment of how capable a process parameter is of meeting its specification limits. Typically, a process with a Cpk equal to or greater than 1.33 can consistently meet its specification limits, while a process parameter with a Cpk equal to or lower than 1.0 cannot. Using historical data, the team can calculate Cpk values for process steps and uncover potential candidates for improvement, as in Figure 3, below.
More advanced statistical tools such as regression analysis and design of experiments can subsequently be used to establish the causal relationships and understand interactions between a finished product’s release parameters and other in-process parameters and raw material characteristics.
Statistical predictive modeling, one of the most sophisticated and powerful of these tools can use either retrospective data or prospective experimental data to “simulate” actual process performance. For example, prior to making the change on the floor, an improvement team might use statistical predictive modeling to predict how a change in spray rate, or any other operating parameter, will affect dissolution. This type of quantitative modeling also yields what is called a “table of effects,” which rank orders the contribution of the factors that drive variation in any given release parameter (also known as the response). It also helps identify the critical process parameters which are also the key drivers of variation — the parameters to which you would apply PAT technology.
The catch to all of this is that it requires companies to invest in the skills to effectively utilize these tools and techniques. When a company is early on the path to process understanding it is often helpful to enlist the help of consultants experienced in the use of these tools. The vision, however, should be for every pharmaceutical company to recruit people with these skills.
All of these successive qualitative and quantitative studies should lead to a maximum level of improvement in process understanding. You can then use the resulting process understanding to continuously improve process control, improve manufacturing productivity, improve operator effectiveness, and more. There are millions of dollars to be saved and competitive market positions to be solidified. PAT, and more specifically the concept of process understanding, holds out the promise of transforming those pharmaceutical companies that choose to fully embrace the concept.
About the Author
Ray Schneider is Tunnell Consulting’s Chief Consulting Officer, Vice President and Director of the Process/Organizational Excellence practice.