Recently, at an event that it hosted for the American Chemical Society and the American Institute for Chemical Engineers, Abbott Laboratories (North Chicago, Ill.) shared some of the best practices that guide the way it designs and operates potent drug processing facilities.
Included was a tour of a new oral dosage form R&D facility, which is expected to open later this year. Built using modular construction, the new facility will include areas where new drugs ad active ingredients will be evaluated, clinical-trial-scale quantities of new compounds will be manufactured, including some highly active materials. Facilities for processing active or toxic drugs feature containment that reduces exposure levels to 0.2-microgram-per-m3.
Because some of Abbotts most promising new therapies, including some cancer treatments, involve the use of active or toxic ingredients, the new facility includes HAPI processing areas, where all process equipment, including mills and filter-dryers, is contained and customized to minimize the need for operators to interact with product or raw materials, through features such as butterfly valves and IBC lifts and disposable equipment (see photo at right). Abbotts ultimate goal is a shirt-sleeve environment where employees wont have to wear personnel protective equipment (PPE) unless absolutely necessary.
Extending this hands off theme is an online NIR and a particle size analyzer, which applies Process Analytical Technology (PAT) to assess material quality during manufacturing.
The new facilitys design reflects the work of Abbotts Potent Drug Containment Program, which has applied advanced containment principles to manufacturing facilities in Puerto Rico, Ireland and the U.S., and is now using them in the companys Global Pharmaceutical Research & Development facilities. Headed by David McAlonan, senior program manager, Abbott Global Engineering Services, the program involves a cross-disciplinary team of 75 people, including representatives from corporate and environmental health and safety, QA, planning, validation, and the business side.
The team began its efforts by benchmarking and consulting with others in the industry to determine the best containment practices.
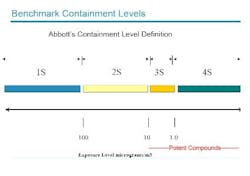
Over the next few years, the program will undertake about 20 potent drug containment projects, for facilities ranging from labs processing sub-kilogram quantities of material, to pilot plants manufacturing up to 75 kilograms. It will also address tech transfer to commercial facilities beginning at the 300-kilogram scale. The company defines potent materials as those that produce clinical effects in adults at a dose of 10 mg/day or less (see Figure 1 below). Employee exposure limits are <10 mg/m3.Employee safety, particularly the prevention of aerosol dust inhalation, is the primary driver for containment projects at Abbott, explained Howard Morton, director of process chemistry and global pharmaceutical research and development. Abbott focuses on engineering controls, procedures and practice as primary protective measures, and divides process areas into risk categories based on the inhalation hazards posed. Disposable equipment is preferred whenever possible, Morton said, even in moderately hazardous 2-S environments.
Controlling the process is critical to minimizing impurities and the number of processing steps, both of which also help improve employee safety. Since close control of particle size will be critical in optimizing the bioavailability of Abbotts new drugs, a decision was made to use PAT to help improve control particle size and product quality. For example, one new compound that will be made at the facility comes in two polymorph forms one shape is desired, the other isnt. Using NIR and particle size analysis, the R&D team can monitor the generation of both polymorphs and adjust conditions to maximize output of the desired form. The PAT installation has eliminated the need for off-site lab sampling. In-situ monitoring is the way we want to go, said Morton.
Safety features at the new facility include:
- Separate personnel and material airlocks, with decontamination showers in personnel airlocks.
- Document isolators in potent suites, since paper cant be decontaminated. Paperless technologies are being evaluated.
- Continuous monitoring of environmental conditions such as relative humidity, temperature and pressure are rigorously monitored.
- One-through air, circulated 20 times per hour, with HEPA supply and return.
- Negative pressures in processing areas.
- Bag in/Bag Out HEPA, accessed from the potent process side.
- Contained drain systems (in the past, these were typically closed and connected to a containment tank).
Since mechanical support will be critical, the ratio of processing to mechanical support area at the new facility will be about 1 to 1.5, McAlonan said.
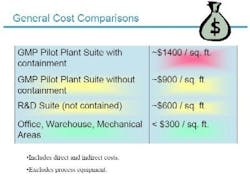
Given the costs of building containment facilities today (see Figure 2 below), it can often be difficult to convince senior management to invest in such facilities, but improved safety and productivity made a strong case for the program, and the new building.
Ergonomics drove facility design. First, a plywood mockup for isolators was built to ensure that operators would be comfortable using the equipment. Some isolators have been placed on tables that can be adjusted to different operator heights. Models were also used for gowning areas, and people tried them out.
The team also used 3-D modeling to optimize the placement of skids, IBC and valves.