Virtual Sensors for Advanced Pharmaceutical Process Control
Real-time information on key process variables is a prerequisite to greater manufacturing efficiency and improved process control. Without this information, quality and efficiency are hampered, resulting in product loss, rework and higher manufacturing costs.
In order to control quality and process variables, however, one must accurately and continuously measure process conditions. In theory, conventional analytical hardware can provide this information, but existing sensors cannot measure some types of data online in real time. Even sensors that work online can be unreliable, and readings can “drift.”
“Soft” or “virtual” sensors offer a way around the limitations of analytical hardware. As their name implies, these are pieces of software code, developed by taking process data readings and modeling the process.
Most drug manufacturers have not yet explored virtual sensing, just as they have not yet harnessed advanced process control technologies such as distributed digital control systems or advanced control and optimization algorithms. Technologies using smaller and more powerful microchips and faster computers have already improved the manufacturing efficiency for the petrochemicals, cement, steel, automotive and other industries. Most drug makers today still rely on off-line quality testing in the laboratory, leading to delays, high cycle times and production costs.
FDA’s process analytical technologies (PAT) framework [1] allows drug manufacturers to use more advanced process control strategies to achieve “quality by design” for their products and processes. Virtual sensors, programmed using process measurements, allow drug makers to understand, manage and control all critical sources of process and product variability, thus achieving PAT’s primary goal.
This article will examine virtual sensing and highlight its application in the drug manufacturing industry by providing relevant and practical examples from other industries.
Applying virtual sensors
Virtual sensors estimate the value of primary variables that are impractical, or impossible, to measure online, such as particle size distribution, melt-flow index, composition or flooding in a distillation column. The sensors use temperature, pressure, flow rate and other variables that can be reliably and inexpensively measured online, to derive these values.
Virtual sensors thus provide a solution where process parameters are difficult to measure in real time, or where process conditions would damage hardware (for example, highly corrosive atmospheres). They can also be used in situations where an analytical device cannot be linked with real-time data acquisition and control systems.
By continuously estimating critical process parameters from continuous data readings, virtual sensors allow for online “measurement” of these parameters, which can be used as feedback for timely and effective process control. Once developed and tuned, virtual sensors can be applied for online control, process monitoring and fault diagnostics.
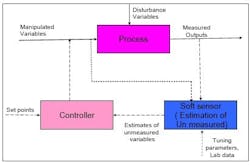
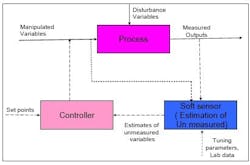
Figure 1, below, illustrates the manner in which a virtual sensor might be used for online applications. The application runs in real time, parallel to the process. The virtual sensor is subject to the same changes and inputs that take place in the actual process. Any information that is not available online, such as data on feed-composition changes, can still be fed into the virtual sensor through the user interfaces provided in the application.
Thus, the model in the virtual sensor receives live data from the plant, and continuously computes all relevant data points. Outputs are stored in the same real-time database and are available, along with signals from field sensors, for feedback control or any other monitoring purposes.
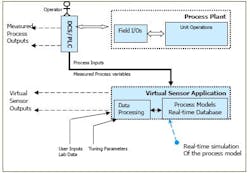
Once a virtual sensor has been developed and validated, it can be used for various purposes such as online monitoring and diagnostics, or feedback in advanced process control. Figure 2, below, demonstrates the application of virtual sensors in process control.
Developing models
At the core of any successful virtual sensor application is a well-designed and well-tuned model of the underlying process — a model that presupposes in-depth knowledge of the physical phenomena underlying the process.
Different types and combinations of models can be used for different applications, depending on the application and the complexity of the process. The most common models used for the development of virtual sensors include:
- Phenomenological
- (first principle-based);
- Data-based,
- employing:
- Univariate or multivariate statistical analysis techniques such as regression-based, principal component analysis (PCA), and partial least squares (PLS);
- Artificial neural networks, of either feedforward or recurrent variety;
- Advanced signal processing techniques such as wavelets and wavenets;
- Fuzzy models.
- Gray-box:
- a combination of phenomenological and data-based models.
References to online resources describing these technologies mentioned above are provided in the reference list below [2-6].
Tuning is essential to ensure that the sensors provide reliable information, since even the most carefully developed model may not capture all the nuances of process behavior under all possible circumstances. It is typically done by taking periodic laboratory analyses of the critical-to-quality variables, and using them to adjust the model itself or refine the virtual sensor estimates.
PAT and virtual sensors
With PAT, FDA is encouraging a shift from the industry’s reliance on feedback or corrective action, after the product has been made, to continuous quality verification and control during production. Online measurements are preferred, because they minimize potential sampling errors and provide continuous rather than sample-based quality verification. Infrared (IR), near infrared (NIR), nuclear magnetic resonance (NMR) and Raman spectroscopy are all being used for pharmaceutical PAT.
The virtual sensor technology provides huge opportunities for pharmaceutical PAT, in applications such as:
- Dryer monitoring, control and optimization, using outlet moisture estimation as the virtual sensor;
- Particle-size distribution in the crystallization process of API manufacturing;
- Size distribution in granulation process;
- pH in various processes.
Since virtual sensors are easy to connect with both hardware sensors and enterprise IT platforms, they can improve data flow, both horizontally and vertically, within any organization. Distributed control systems (DCS) allow the sensors to connect with hardware sensors, while programmable limit switches (PLS) let them connect with enterprise systems.
To risk-averse pharmaceutical companies, virtual sensors might appear to be a radical technology, but they have already proven their worth in other industries. The following examples from the mining, automotive and cement making industries explain how they might be applied in pharmaceutical manufacturing.
Particle size distribution
In this case, a virtual sensor was developed from first principle models, and used in the wet grinding circuit of a lead-zinc ore beneficiation plant. In the first step of the process, the ore is pulverized into finely ground particles in wet grinding mills to separate valuable material from the associated gangue. The grinding circuit comprises an open-circuit rod mill, a closed-circuit ball mill, and a two-stage classification circuit with hydro cyclones.
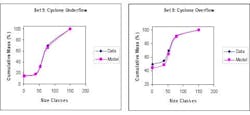
This process was difficult to control in real time, because particle sizes could not be measured in real time. Instead, a virtual sensor was used to estimate the product’s particle size distribution, throughput and the percent of solids in the circuit outlet. The estimates were then used for feedback in advanced process control. Figure 3, below, shows the performance validation plots for size classes based on the plant data.
A similar virtual sensor could easily be developed for pharmaceutical tablet manufacturing. In such an operation, the milling step is crucial as it delivers uniformly sized API before blending. A virtual sensor could be applied for PSD monitoring in sizing and milling, greatly improving productivity.
Virtual sensors for raw cement mix quality parameters
This is an adaptive, dynamic virtual-sensor model based on mass-balance first principles, and was customized for the soft sensing of the raw mix (raw meal) quality in a cement plant. The model uses some heuristics-based adjustments to handle the uncertainties in feed-stream composition, and also has some heuristics/logic built into it to make it viable for industrial use.
The output parameters of the virtual sensor are three main quality parameters of the raw material mix (raw meal) — namely, Lime Saturation Factor (LSF), Alumina Modulus (AM), and Silica Modulus (SM). These quality parameters are dependent on the cement composition (oxides — CaO, Al2O3, Fe2O3, and SiO2). The virtual sensor predicts the mill outlet material composition and based on these predictions, creates the quality parameters LSF, AM and SM. The virtual sensor model is adapted based on changes in process variables to handle the nonlinearity of the process. The virtual sensor estimates are adjusted based on the availability of actual laboratory measurements.
This virtual sensor is already used in the industrial implementation of the raw mix optimizer — the online supervisory ratio (proportioning) control solution for the raw mix process in the cement industry. Figure 4, below, shows the performance validation plots for LSF, AM and SM based on the plant data.
A similar process in pharmaceutical manufacturing is the blending operation, where the milled API is mixed with a number of inactive ingredients with the aim of delivering uniformly blended material for compression.
Virtual sensors for air-fuel ratio
This example relates the use of virtual sensors in the automobile industry. Today’s stringent emission regulations require that cars maintain precise air-fuel ratio (AFR) control under varying driving conditions. A major automaker saw a pressing need to replace current hardware emission/AFR sensors, which it considered costly and unreliable. It developed a real-time operable virtual sensor that uses engine parameters already measured on board the vehicle. The virtual sensor relied on artificial neural networks.
These examples indicate the application of virtual sensors in pharmaceutical manufacturing. Regardless of the industry, virtual sensors help improve process understanding and provide a powerful tool for managing process and product variability. The technology only awaits application in the drug manufacturing domain.
References
- Guidance for Industry: PAT — A framework for innovative pharmaceutical development, manufacturing and quality assurance: www.fda.gov/cder/guidance/6419fln.pdf
- An Introduction to Fuzzy Logics: www.seattlerobotics.org/encoder/mar98/fuz/fl_part1.html
- Principal Component Analysis: www.ucl.ac.uk/oncology/MicroCore/HTML_resource/PCA_1.htm
- Partial Least Squares: www.statsoft.com/textbook/stpls.html
- An Introduction to Neural Networks: www.cs.stir.ac.uk/~lss/NNIntro/InvSlides.html
- Advanced Process Control: http://lorien.ncl.ac.uk/ming/advcontrl/sect1.htm
About the Authors
N.C. Chakrabarti has more than 12 years of professional experience in simulation, optimization, process control, design and process engineering, commissioning and operations of process plants in the chemical and petrochemical industries. He is currently working in the Advanced Process Control group in the Plant Automation Services Practice of TCS. A postgraduate from the Indian Institute of Technology, Kharagpur in Chemical Engineering, he also holds a bachelor’s degree in Chemistry from Calcutta University.
Rajesh Sahasrabudhe has more than 12 years of professional experience in development and implementation of manufacturing IT systems. Rajesh is currently the head of the Advanced Process Control subpractice that is part of the Plant Automation Service practice of TCS. Rajesh has done his graduate work in Instrumentation Engineering at the University of Pune, and has a post-graduate degree in Controls and Instrumentation from the Indian Institute of Technology, Powai, Mumbai.
Ravindra Bhuyarkar is an engineering graduate and has an MBA in Finance and Marketing. Ravi has a technology background in embedded systems applications, networking and interconnectivity in industrial automation and building automation and has over 10 years of experience in sales and business development. Currently, he is head of the Marketing division for the Plant Automation Services.