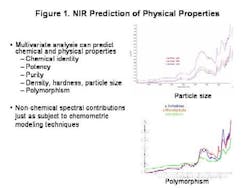
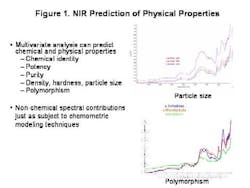
The last few years, and the knowledge that the industry spends over $90 billion each year on manufacturing alone, have brought increased regulatory attention to the importance of controlling the pharmaceutical manufacturing process. FDA's PAT Initiative and the resulting Guidance to Industry promises to play a key role in pushing pharmaceutical manufacturing into the 21st century.To start off any PAT program on the right track, and to gain the process understanding required, pharmaceutical manufacturers must start at the very beginning. They must understand the impact that varying raw material quality has on the final product.Existing techniques only address part of the raw material quality picture. Traditional approaches to determining raw material quality are based on identity, chemical purity and pharmacopeial methodologies. These methods are all useful and important, but in most pharmaceutical processes, they are not enough because they cant address physical changes in the product. Such changes are extremely important in an operation such as tablet manufacturing, in which powdered materials are handled, mixed, modified and compressed, changing their physical attributes.Its not unusual for OOS tablet batch failures to be traced back to a problem with a raw material ingredients physical property. This occurs, even when that raw material passed all the traditional inspection processes in place for incoming materials.NIRs Role in Quality AssuranceAlready well established as an analytical tool for pharmaceutical testing, FT-NIR can provide information on both chemical quality attributes and physical properties quickly, easily and without destroying the sample.The technique can be used to create a raw material information database containing information on both chemical and physical properties, and batch-to-batch variance. Use of the technique can also speed up quality testing, eliminating the time spent waiting for results, and reducing inventory and the need for raw material quarantine.When part of a properly designed PAT effort, the analytical method offers fast, repeatable and reliable results. Once an NIR raw material database has been set up, links between quality performance later in the process, and FT-NIR spectra can often be established.From these links, causal relationships can be explored and investigated, or the system can be deployed to screen incoming raw materials to determine how suitable they are for the given process. Thus, raw material qualification via FT-NIR can become the first critical control point in the process, enabling the use of more advanced process control techniques.Important points to consider are first and foremost, whether the material is the correct one. Does it meet specifications for purity or water content? Is it useable in the process, given its chemical and physical properties? Often, the same API in a form with a different shape or particle size, can lead to surprising product failures.FT-NIR can help manufacturers address all these issues, allowing users to predict physical characteristics by analyzing spectra and the reference method for the trait. It also allows manufacturability to be predicted based on trait and output data.Sampling Raw MaterialsFiber optics is often used for chemical identification. It is typically applied in the laminar flow area or through the bag. Its suitable for chemical but not necessarily physical characteristic qualification.Quality is often a reflection of physical processes, and the impact of granulation, blending and compression. FT-NIR determines chemical identification but also studies particle size and distribution, polymorphism and water content, determining moisture sorption, potential for recrystallization, overall surface, flow and granulation properties. Combined with multivariate analysis and modeling, it can predict puritym density, hardness, particle size and polymorphism. Ultimately, it can determine the materials impact on compression, dissolution and content uniformity.Lets consider particle size, which determines a powders flowability, and thus its performance in blending operations, but also influences granulation and compression. Where traditionally particle sizing is a game of averages, FT-NIR raises it to the level of a science, verifying average particle size distribution information as well as nonchemical spectral information (Figure 1, below).The technology also allows analytics to be applied, effectively, to the overall process, so that materials can be tracked via a lot number throughout the process, using RFID or barcoding, an ERP field or link to parameter value or XML or other data input. For facilities that use MES software, the platform can read the parameter value, and set the recipe according to the understood relationships that have been programmed. Variable processes can be further controlled through downstream analytics (specifically, third order feed-forward control).Eventually, an effective PAT system will become as much a part of your companys mission-critical manufacturing infrastructure as your blender or tablet press. FT-NIR can be an effective tool in any PAT program.The technology neednt be implemented all at once, but can be utilized in stages: at the most basic level, to detect defective raw materials early and cheaply. Later it can be used to map and direct materials to different processes using a first order feed-forward mechanism. Subsequently, it can be used in a second order mechanism to adjust processes based on early phase information for example, to adjust granulation time based on excipient particle size, or to set pre-blend parameters based on magnesium stearate properties.In short, the FT-NIR technologys power justifies its growing presence in pharmaceutical PAT initiatives. But more of these initiatives would do well to focus on raw materials, where much of the product variability starts.
About the Author
Paul Entrop
Product Team Director
Sign up for our eNewsletters
Get the latest news and updates