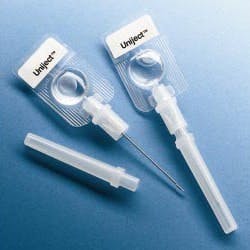
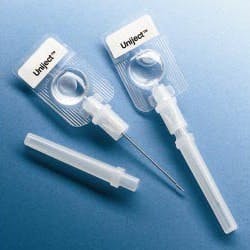
The World Health Organization (WHO), the Red Cross and the Safe Injection Global Network (SIGN) are supporting programs to improve injection safety worldwide. In developing nations, 10 billion syringes are used each year, but unfortunately, unsafe injection practices are common and cause at least 1.3 million deaths and 10 million cases of hepatitis annually.
WHO recommends single-use, auto-disable (AD) syringes and is attempting to eliminate the use of traditional syringes as soon as possible. A worldwide effort to improve injection safety is being coordinated by SIGN with the guiding principle that a safe injection should not harm the recipient, the healthcare worker administering the injection, or the community at large.
Abandoning traditional barrel-plunger-needle syringes in favor of auto-disable technologies offers an effective way to solve a major problem that is difficult to control procedurally. However, the new designs will require efficient device manufacturing technologies, effective sterile filling equipment and extensive testing.
Although new designs have not yet penetrated the market, purchasing contract requirements by large organizations like UNICEF or PAHO may force parenterals suppliers to adopt new technologies. A proactive approach, and close evaluation of alternatives, can pay off.
This article will review hollow-needle type AD injection devices, and discuss the manufacturing issues and challenges they pose.
Scoping the problem
In developed nations, disposable plastic syringes have been in widespread use for 30 years, virtually eliminating re-use in a professional medical setting.
In developing nations, however, the cost of a new, sterile syringe and needle is often enough to prevent the use of vaccines and legitimate therapeutic drugs. When the vaccine or drug is available in a multidose form, economic realities mean that all too often unclean and unsterilized syringes and needles will be reused, and spread disease.
Some solutions to this problem are procedural. However, technical solutions, such as the following, promise to be much more effective:
- Alternative injector design and methodology
- Syringe plungers that lock once used
- Plastic liquid compartments that collapse on use
- Needles that can be used only once
- Needle-free injectors based on high-velocity sprays these can be extremely cost-effective, but permit aerosolization of the recipients bodily fluids, creating the possibility of cross-contamination [3].
- Oral, inhaled or skin administration, via patches or inhalers an approach that, other than its notable success with the oral polio vaccine, hasnt worked with many vaccines.
- Product compatibility, leachability and stability studies, which must be performed on each product formulation;
- Container integrity testing covering the range of temperatures and pressures that the final product may encounter;
- If there is an applied label, adhesiveness studies and penetration or migration of adhesive components into the container and product must be performed at all manufacturing and storage temperatures and humidities, often with real-time stability studies that may last several years;
- Container closure integrity and microbial ingress must also be evaluated before a product can be launched in a new container.
- Simonsen, L., et al. Unsafe Injections in the Developing World and Transmission of Bloodborne Pathogens: A Review. Bull. WHO, 77:789-800, 1999.
- Hutin, Y. and R. T. Chen. Injection Safety: A Global Challenge. Bull WHO 77: 787-788, 1999.
- Hutin, Y. and W. Dierick, WHO-ISO collaboration for the development of international standards for safer injection technologies, WSC Workshop, Geneva, Feb. 2004.
- Ekwueme, D. U., B. G. Weniger and R. T. Chen. Model-based estimates of risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa. Bull WHO, 80:859-870, 2002.
- Godal, T., Presentation at the 4th GAVI Board Meeting, December, 2004.
- Lloyd, J.S. and J.B. Milstein, Auto-Disable Syringes for Immunization: Issues in Technology Transfer, Bull. WHO, 77:1001, 1999.
- Dubin, C., Drug Discovery and Delivery: Tear Down Those Walls!, Drug Delivery Technology, 5: 28-32, 2005.
About the Author
Dr. Gerson is principal of Axenic, Inc., a global consulting firm specializing in process development, manufacturing, facility design and cGMP operations for the biopharmaceutical industries. He has worked as managing director for manufacturing and development at the International AIDS Vaccine Initiative, VP of manufacturing and development at Acambis, where he developed the process for and manufactured over 200 million doses of Smallpox vaccine. Prior to that, he worked as managing director of vaccine manufacturing at Wyeth-Lederle Vaccines, and before that, as VP of R&D at Apotex Fermentation, Inc., and as Assistant VP of manufacturing and process development for Connaught Laboratories in Toronto.
He has a Ph.D. in Biophysics from McGill University, has taught at the University of Western Ontario, and worked as an independent researcher at the Basel Institute for Immunology.